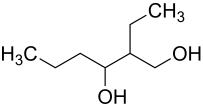
2-ethylhexane-1,3-diol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| Structural formula without information on stereoisomerism | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 2-ethylhexane-1,3-diol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 18 O 2 | |||||||||||||||||||||
| Brief description |
colorless and odorless liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 146.22 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.94 g cm −3 |
|||||||||||||||||||||
| Melting point |
−40 ° C |
|||||||||||||||||||||
| boiling point |
243 ° C |
|||||||||||||||||||||
| Vapor pressure |
<0.01 hPa (20 ° C) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.4497 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
2-Ethylhexan-1,3-diol (also simplified ethylhexanediol ) is a mixture of several isomeric organic chemical compounds from the group of alkanediols .
Stereochemistry
The molecule has two stereocenters , on the 2nd and 3rd carbon, so that there are four stereoisomeric compounds of ethylhexanediol:
- (2 R , 3 R ) -2-ethylhexane-1,3-diol
- (2 S , 3 S ) -2-ethylhexane-1,3-diol
- (2 R , 3 S ) -2-ethylhexane-1,3-diol
- (2 S , 3 R ) -2-ethylhexane-1,3-diol
presentation
Ethylhexanediol can be synthesized by condensation of butanal with magnesium aluminum ethoxide and subsequent hydrolysis of the ester formed . Another approach is the hydrogenation of butyraldol ( 2-ethyl-3-hydroxyhexanal ). Starting compounds of industrial production are chemicals of the petrochemical industry .
use
It is used as a mixture of the four stereoisomeric forms as a repellent against insects (especially effective against Aedes ), cooling lubricant and as an emollient .
See also
Individual evidence
- ↑ Entry on ETHYL HEXANEDIOL in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ a b c d e f g h i j k Entry on 2-ethylhexane-1,3-diol in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b c Werner Baumann, Thomas Rothardt: printing chemicals . Springer, Berlin / Heidelberg 1999, ISBN 3-540-66046-1 , pp. 935 ( limited preview in Google Book search).
- ↑ Entry on 2-ethylhexane-1,3-diol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Military Preventive Medicine: Mobilization and Deployment, Vol 1.Chapter 22: Personal Protection Measures Against Arthropods , p. 508.
