3,5-dibromosalicylic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 3,5-dibromosalicylic acid | ||||||||||||||||||
| other names |
3,5-dibromo-2-hydroxybenzoic acid |
||||||||||||||||||
| Molecular formula | C 7 H 4 Br 2 O 3 | ||||||||||||||||||
| Brief description |
long colorless needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 295.91 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
226-229 ° C |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
3,5-Dibromosalicylic acid is an organic chemical compound that belongs to the group of phenols as well as to the group of aromatic carboxylic acids . It is therefore a phenolic acid .
presentation
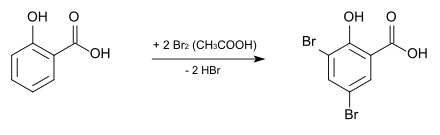
3,5-Dibromosalicylic acid can be produced from salicylic acid by bromination with elemental bromine in glacial acetic acid .
use
The copper salt of 3,5-dibromosalicylic acid is used as a fungicide and as a bactericide . Furthermore, 3,5-dibromosalicylic acid is the starting material in the synthesis of antibiotics .
Reactions
Further bromination of 3,5-dibromosalicylic acid leads, after decarboxylation, to 2,4,6-tribromophenol , which in turn reacts further with bromine to form 2,4,4,6-tetrabromo-2,5-cyclohexadienone. This reaction can be reversed by hydrogen iodide .
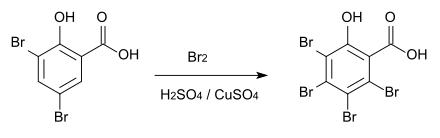
However, if the reaction is carried out in sulfuric acid with copper sulfate as a catalyst , 3,4,5,6-tetrabromosalicylic acid is obtained, which decomposes above 230 ° C.
links
The barium salt of 3,5-dibromosalicylic acid forms crystals with the composition (C 7 H 3 Br 2 O 3 ) 2 Ba · 4 H 2 O, the lead salt is insoluble in water.
Individual evidence
- ↑ a b E. Lellmann, R. Grothmann: Ueber some derivatives of salicylic acid , in: Chem. Ber. , 1884 , 17 , pp. 2724-2731; doi : 10.1002 / cber.188401702221 .
- ↑ a b Entry on 3,5-dibromosalicylic acid at ChemBlink , accessed on February 25, 2011.
- ↑ a b Data sheet 3,5-Dibromosalicylic acid from Sigma-Aldrich , accessed on May 9, 2017 ( PDF ).
- ↑ a b c B. Renneberg, M. Kellner, H. Laatsch: Synthesis of halogenated benzyl and benzoyl pyrroles , in: Liebigs Ann. Chem. , 1993 , pp. 847-852. doi : 10.1002 / jlac.1993199301134 .
- ↑ US Patent 399034: Biocidal 3,5-dibromosalicylic acid salts .
- ↑ John A. Price: The Structure of Tribromophenol bromide , in: J. Am. Chem. Soc. , 1955 , 77 (20), pp. 5436-5437; doi : 10.1021 / ja01625a081 .
- ↑ Hans P. Latscha, Helmut A. Klein, Gerald W. Linti: Analytical Chemistry: Chemie-Basiswissen III , p. 287 ( limited preview in the Google book search).