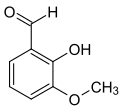
ortho- vanillin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
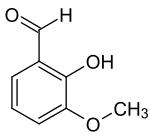
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | ortho- vanillin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 8 O 3 | ||||||||||||||||||
| Brief description |
light yellow crystalline needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 152.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
43-45 ° C |
||||||||||||||||||
| boiling point |
265-266 ° C |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
ortho- vanillin ( 2-hydroxy-3-methoxybenzaldehyde ) is an organic chemical compound with the empirical formula C 8 H 8 O 3 . It is a derivative of benzaldehyde with an additional hydroxyl and methoxy group . The prefix ortho indicates the position of the hydroxyl group in relation to the aldehyde group.
History and occurrence
ortho- vanillin was first discovered by Ferdinand Tiemann in 1876 . It is found in extracts and essential oils of many plants. In 1910, Francis Nölting developed methods for the preparation of pure substances, and he was also able to demonstrate the versatility of this compound as a synthetic precursor for a large number of compounds such as coumarins . In 1920 the compound was used to dye animal hides. However, it is not in great demand in food and is therefore a less frequently produced and found additive.
properties
Physical Properties
ortho- vanillin forms light yellow crystalline needles. It melts at 43–45 ° C and boils at 265–266 ° C. It is soluble in THF , ethanol and methanol . It crystallizes in the orthorhombic crystal system in the space group Fdd 2 (space group no. 43) with the lattice parameters a = 2509.9 pm , b = 2452.2 pm, c = 479.1 pm and 16 formula units per unit cell .
Chemical properties
It differs significantly from its isomer , vanillin, because, in contrast, it does not have the characteristic and intense smell of vanilla. The prefix ortho indicates the position of the hydroxyl group in relation to the aldehyde group; in vanillin these two groups are in para position.
ortho -vanillin can be converted into ortho-vanillic acid by melting with potassium hydroxide . This in turn can form 2,3-dihydroxybenzoic acid with hydrogen bromide .
Biological properties
ortho- vanillin is a weak inhibitor of tyrosinase , and shows both antimutagenic and comutagenic properties in Escherichia coli . It possesses moderate antifungal and antibacterial properties.
See also
Individual evidence
- ↑ a b c F. Iwasaki, I. Tanaka, A. Aihara: “2-Hydroxy-3-methoxybenzaldehyde ( o- vanillin)”, in: Acta Cryst. B , 1976 , 32 (4), pp. 1264-1266; doi : 10.1107 / S0567740876005086 .
- ↑ a b c d e f g data sheet o-vanillin from Sigma-Aldrich , accessed on April 16, 2011 ( PDF ).
- ↑ a b Solubility of ortho-vanillin in non-aqueous solvents
- ↑ Ferdinand Tiemann: "About the compounds belonging to the coniferyl and vanillin series", in: Reports of the German Chemical Society , 1876 , 9 , pp. 409-423; doi : 10.1002 / cber.187600901133 ( digitized on Gallica ).
- ^ AH Abou Zeid, AA Sleem: "Natural and stress constituents from Spinacia oleracea L. leaves and their biological activities", in: Bulletin of the Faculty of Pharmacy (Cairo University) , 2002 , 40 (2), pp. 153-167 .
- ^ Jean-Christophe Barbe, Alain Bertrand: “Quantitative analysis of volatile compounds stemming from oak wood. Application to the aging of wines in barrels “, in: Journal des Sciences et Techniques de la Tonnellerie , 1996 , 2 , pp. 77-88.
- ^ EJ Brunke, FJ Hammerschmidt, G. Schmaus: "The essential oil of Santolina chamaecyparissus L. (Santolina chamaecyparissus essential oil)", in: Parfümerie und Kosmetik , 1992 , 73 (9), pp. 617-618, 623-624 , 626, 628-630, 632, 634-637.
- ^ Francis AM Noelting: "o-Hydroxy-m-methoxybenzaldehyde (Orthovanillin)", in: Annales de Chimie et de Physique , 1910 , 19 , pp. 476-550.
- ↑ Otto Gerngross: “The colorations of animal skin by o-vanillin and o-protocatechualdehyde and aldehyde tanning”, in: Angewandte Chemie , 1920 , 33 (44), pp. 136-138; doi : 10.1002 / anie.19200334403 .
- ^ VK Ahluwalia: Intermediates For Organic Synthesis . IK International Pvt Ltd, 2005, ISBN 978-81-88237-33-3 , pp. 76 ( limited preview in Google Book search).
- ↑ Isao Kubo, Ikuyo Kinst-Hori: “Tyrosinase inhibitory activity of the olive oil flavor compounds”, in: Journal of Agricultural and Food Chemistry , 1999 , 47 (11), pp. 4574-4578; doi : 10.1021 / jf990165v .
- ↑ Kazuko Watanabe, Toshihiro Ohta, Yasuhiko Shirasu: “Enhancement and inhibition of mutation by o-vanillin in Escherichia coli”, in: Mutation Research , 1989 , 218 (2), pp. 105-109; PMID 2671704 .
- ↑ Kazuhiko Takahashi, Mutsuo Sekiguchi, Yutaka Kawazoe: "A specific inhibition of induction of adaptive response by o-vanillin, a potent comutagen", in: Biochemical and Biophysical Research Communications , 1989 , 162 (3), pp. 1376-1381; PMID 2669748 .
- ↑ I. Leifertova, N. Hejtmankova, H. Hlava, J. Kudrnáčova, F. Šantavý "Antifungal and antibacterial effects of phenolic substances. A study of the relation between the biological activity and the constitution of the investigated compounds ", in: Acta Universitatis Palackianae Olomucensis, Facultatis Medicae , 1975 , 74 , pp. 83-101.