Cellulose acetate
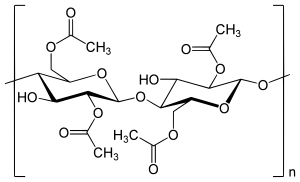
Cellulose acetate ( abbreviation CA , formerly acetyl cellulose ) is a collective name for the acetic acid esters of cellulose . By the action of glacial acetic acid and acetic anhydride on cellulose (mostly cellulose or cotton linters ) in the presence of catalysts ( sulfuric acid or zinc chloride ), the cellulose triacetate (abbreviation CTA ), the so-called primary acetate, must first be produced, in which the three hydroxyl groups per glucose building block are esterified. This is necessary because a partial esterification leads directly only to mixtures of not and completely acetylated cellulose. However, since cellulose triacetate is unfavorable for most applications because of its limited solubility and poor compatibility with plasticizers, the addition of water is used to partially saponify the cellulose triacetate, whereby acetic acid contents of 41 to 62.5% can be set in the ester depending on the temperature and exposure time. Various types of secondary acetates (e.g. 2½-acetate and diacetate) are thus obtained. Depending on the degree of esterification , the viscosity of the cellulose acetate types changes (the higher the degree of esterification, the higher the viscosity), which means that a wide range of properties and thus a wide range of products can be achieved. This ranges from electrical insulation foils to fibers for textile purposes and fiber cables for cigarette filters to low-viscosity additives for adhesives and textile auxiliaries .
Cellulose acetate is one of the oldest thermoplastics and, as a derivative of the natural substance cellulose, is counted among the bio-based plastics , which in their fiber form were previously referred to as semi-synthetic fibers.
history
The period from the first presentation to the first large-scale production and application ranged from 1865 to around the beginning / middle of the 1920s.
- In 1865 Paul Schützenberger produced cellulose acetate for the first time by heating cellulose in the form of cotton fibers with acetic anhydride in a closed glass tube to 130 ° C to 140 ° C until it completely dissolved. After precipitation with water, washing and drying, it obtained a white amorphous powder which was insoluble in water but soluble in alcohol and concentrated acetic acid.
- In 1879, the acetate process was further developed by Antoine Paul Nicolas Franchimont , who found out that the acetylation of cellulose is catalyzed by sulfuric acid or zinc chloride.
- In 1894, Charles Frederick Cross and Edward John Bevan applied for British Patent No. 9676 to usher in a new period in development, as this patent first mentioned the industrial value and technical application of cellulose acetate. In the process described, cellulose hydrate and acetyl chloride were heated in the presence of a catalyst ( zinc chloride ) and, for the first time, larger amounts of cellulose acetate, which was soluble in chloroform , were obtained. By evaporating the solvent, coherent skins could be obtained, which is why the inventors proposed cellulose acetate as a replacement for collodion in pharmacy and surgery. The patents were acquired from Prince Guido Henckel von Donnersmarck and expanded with his own patents, so that on their basis the first cellulose acetate was produced in the Fürst Guido Donnersmarck artificial silk and acetate works in Sydowsaue near Stettin (today Szczecin- Żydowce , Poland) in 1898 could be factory-made. The first, albeit non-marketable, cellulose acetate silk was later produced there in a test facility after Fürst Donnersmarck had acquired further patents from Mork, Little and Walker.
- Between 1899 and 1901 Leonhard Lederer applied for patents which also laid the foundations for the industrial production of cellulose acetate, whereby hydrate cellulose still had to be assumed, which remained problematic. However, the use of acetic anhydride and sulfuric acid made it possible to achieve a considerably lower reaction temperature than the previous process.
- 1901 started a research group of the paint factories vorm. Friedrich Bayer Co, Elberfeld (now part of Wuppertal) under the direction of Arthur Eichengrün, development work on a process for the production of high-quality acetyl cellulose. It was possible for the first time to produce cellulose triacetate on the basis of normal cellulose, i.e. without the preceding process step of hydration. This cellulose acetate was slightly soluble in chloroform but less soluble in acetone . However, the simplified production of cellulose triacetate did not meet expectations, so that after years of trials since around 1911, no production, not even experimentally, based on this process concept has taken place.
- In 1904 the American George Wellington Miles applied for a patent in the USA and in 1905 in Europe, which describes the production of an acetone-soluble cellulose acetate. However, he did not succeed in carrying out the process on a large scale, and the acetate produced was of inferior quality due to severe cellulose fiber degradation. In 1904, BASF also became active in this area. In 1902, before the Miles patents became known, the chemists Eichengrün and Becker had already succeeded at Bayer in producing a technically usable, acetone-soluble, non-fragile, storage-stable acetyl cellulose. After years of litigation, Miles sold his German patent to the paint factories vorm. Friedr. Bayer & Co, whose own patent application on this matter was ultimately combined with this patent. The product recognized by the inventors as cellulose hydroacetate was made by the paint factories vorm. Friedr. Bayer & Co. was produced under the brand name Cellit in the following years and used for the formulation of coating compounds for aircraft and airships, but also for the production of coating substrates for a safety film and acetate silk. Acetate silk was first produced in the Jülich artificial silk factory in 1907 after the license was granted for the dry spinning process developed and patented by Eichengrün's research group in 1904 . However, the significance remained minor, since this acetate silk could not be dyed with the dyes known up to then and it was only with the invention of the disperse dyes by René Clavel in 1920 that the wider use of acetate silk was made possible. In the first decade of the twentieth century, Eichengrün's production of cinema films on the basis of flame-retardant cellulose acetate, promoted by Bayer in his work as head of the Düsseldorf Photo Factory, was also hardly of any significance, since, in contrast to the more easily inflammable films based on nitrocellulose, it increased in importance mechanically less durable, more expensive and harder to bond at that time. The introduction of these security films to the market only began step by step after the First World War, and only comprehensively after the Second World War.
- In 1910/11, despite some such failures, the period of increasing factory production of various types of cellulose acetate-based products began. Eichengrün's research and development work, which he carried out in Berlin in his own laboratory from 1909, continued to play a part in this in Germany. He developed z. B. a process that made it possible to produce a celluloid- like product on the basis of cellulose acetate . The Rheinisch-Westfälische-Sprengstoff AG, Troisdorf, produced under license from 1911 the plastic Cellon , which was transparent, non-flammable and splinter-free. This cellon was used for gas masks and aviator goggles, but also for window panes in boat, automobile, airship and aircraft construction. In addition, the production of cellulose acetate varnishes based on Eichengrün's inventions became very important for the developing aircraft and airship industry, where they were used to coat linen and cotton fabrics used as covering. They were manufactured under license by the Berlin company Dr. Quittner Co. himself he produced in his Berlin company “Cellon-Werke Dr. Arthur Eichengrün ”, which emerged from his Cellon laboratory in 1919, primarily used cellulose acetate lacquers for insulation purposes in electrical engineering. The first technically usable injection molding compound developed by Eichengrün on this basis in 1919, which and the products made from it later became known under the brand name Lonarit, was also of great importance for the expansion of the fields of application of cellulose acetate. In 1921, the Berlin master mechanic Hermann Buchholz, in cooperation with Eichengrün, built the first injection molding machine suitable for mass production for plastic molding compounds, on which primarily small mass-produced items such as bobbins for the electrical industry were initially manufactured.
- The Swiss brothers Camille and Henri (Henry) Dreyfus had a significant influence on the development of cellulose acetate production and products made from cellulose acetate of various kinds, who from 1908 concentrated on this with their research and development work and were granted a large number of patents issued by technical standpoints were of great importance for the production of cellulose acetate. It was z. B. from them the fundamental relationship between high viscosity, high tensile strength and elasticity of cellulose acetate. At the end of 1912 the Dreyfus brothers, with the support of the entrepreneur Alexander Clavel-Respinger, founded the Cellonit Gesellschaft Dreyfus & Co. in Basel, where films and coating varnishes based on cellulose acetate, but also the first quantities of acetate silk, were produced. In order to meet the great demand for acetate coatings for the aircraft industry, the British Cellulose and Chemical Manufacturing Co. was set up in Spondon / Great Britain in 1916 with the help of the Dreyfus brothers. In order to continue using the capital-intensive production facilities after the end of the First World War and the associated strong decline in demand for aircraft coatings, the Dreyfus brothers concentrated again on the experimental production of acetate silk. The experiment was successful because they succeeded in developing an improved absorption process to enable almost complete recovery of the solvents produced during production and thus to significantly reduce production costs. At the same time, you were welcomed by the fact that the Swiss René Clavel had found a new dyeing process for cellulose acetate in 1920. The Dreyfus brothers are therefore also regarded as the "fathers" of acetate silk. Starting in 1921, the Spondon site of the company, which has now been renamed British Celanese, Ltd, was initially producing approx. 500 kg per day of acetate silk, which was marketed under the trade name Celanese. Daily production was doubled in the same year and steadily increased over the years. In 1922, large-scale production of Celanese acetate silk began in the USA, and subsequently also in plants in Europe. In Germany, the IG Farben and the Vereinigte Glanzstoff-Fabriken founded AcetA GmbH for the production of cellulose acetate fibers on September 15, 1925. In their expanded plant in Berlin-Lichtenberg, production using the acetate process began in 1927. In the mid-1920s, the introduction of production in key areas of application of the bioplastic cellulose acetate was successfully completed.
Manufacturing
To date, there has been no method of directly producing secondary cellulose acetates. A two-stage synthesis is therefore used, since the attempts at partial esterification of the cellulose result exclusively in a mixture of non-acetylated and fully acetylated cellulose. Cellulose is first always completely converted into cellulose triacetate and then converted into cellulose acetates with a low degree of esterification by hydrolysis.
The production is divided into the following process stages, mostly linked by direct material flow:
- Mechanical processing of the pulp : The pulp, which is usually provided in roll or plate form, is shredded using different types of shredders, e.g. B. fibrillated hammer mill and disc refiner, the successive arrangement of both types of shredder causes optimal resolution.
- Chemical pretreatment : The fibrillated cellulose is treated with acetic acid (if necessary with the addition of small amounts of sulfuric acid) with moderate stirring at 25 ° C to 50 ° C for approx. 1 hour, which leads to continuous evaporation and condensation of the acetic acid in the interstices the fiber particles come. This causes the cellulose particles to swell, which facilitates the diffusion of the solvent particles into these particles during the subsequent process stage. In addition to this acetic acid steam pretreatment, there is also a pretreatment in a thin pulpy state. The pulp is poured into large amounts of water or dilute acetic acid and stirred intensively. Subsequent process steps such as pressing or centrifuging increase the concentration of cellulose in the pulp. At the same time, acetic acid is added in ever higher concentrations. The advantage of this process is that it saves on shredding, as the cellulose layers can be added directly to the stirred tank.
-
Acetylating cellulose : In the commercial production of cellulose acetates, the glacial acetic acid process or the methylene chloride process are usually used for acetylation.
In the glacial acetic acid process, the pretreated cellulose mass is reacted in an acetylation mixture of the solvent glacial acetic acid with an excess of acetic anhydride, which serves as an esterifying agent, and with sulfuric acid as a catalyst, with intensive mechanical mixing. This reaction is strongly exothermic and therefore requires intensive cooling of the reaction vessel. The esterification process is ended by adding water when a highly viscous, clear reaction mixture has formed from the fibrous pulp. This solution (dope) must be gel-free and have the desired viscosity.
In the methylene chloride process, methylene chloride is used as the solvent instead of glacial acetic acid in the acetylation mixture. Since the low-boiling methylene chloride can easily be removed by distillation, process control is very effective even with highly viscous solutions. It can dissolve cellulose triacetate very well even at low temperatures. A small amount of sulfuric acid, but often also perchloric acid, is used as a catalyst. However, acetic acid is usually also formed as a by-product of the reaction, so that the solvent is ultimately a mixture of methylene chloride, acetic anhydride and acetic acid.
A very rare heterogeneous process is the fiber acetate process, which is only used to produce cellulosetricacetate as the end product. The cellulose is suspended in a non-solvent (such as benzene) and esterified with acetic anhydride in the presence of perchloric acid as a catalyst. - Partial saponification (hydrolysis) : In order to obtain the desired secondary cellulose acetate types, the cellulose triacetate obtained is saponified. For this purpose, the triacetate solution is saponified in the presence of an acid catalyst (usually sulfuric acid) by adding water with stirring and heating to usually 60 to 80 ° C. The hydrolysis process is controlled via the concentration of sulfuric acid, the amount of water and the temperature so that the desired molecular breakdown is achieved. The hydrolysis process is then stopped by adding salts (e.g. sodium or magnesium acetate) that neutralize the acid catalyst. The duration of the hydrolysis process depends on the level of the setting parameters.
- Precipitation of the cellulose acetate : When precipitating the cellulose acetate from the reaction solution by means of dilute acetic acid, it is important to obtain uniform and easily washable cellulose acetate flakes. Any methylene chloride present must be completely distilled off prior to precipitation. The acetic acid is then recovered.
-
Washing, drying and mixing of the flakes : Through intensive washing, which usually takes place on the countercurrent principle, the acetic acid must be removed from the flakes down to the smallest traces, otherwise damage ("charring") will occur during the drying process. After the washing liquid has been squeezed out, the flakes are dried to approx. 2-5% residual moisture on a conveyor belt dryer through which hot air flows. For the subsequent production of very high-quality, thermally stable, light-colored and color-stable thermoplastic molding compounds, the cellulose acetate flakes are also bleached and specially stabilized in additional process steps before they are finally dried in order to minimize thermal degradation and discoloration. Before the cellulose acetate flakes are placed in a collecting container, from which they are transported to the corresponding processing plants, the flakes are precisely mixed. This is intended to compensate for deviations in the cellulose acetates from different production batches.
For many further processing processes, the flakes are usually ground into fine powders beforehand. In order to use cellulose acetate in thermoplastic plastics processing methods such. B. to use injection molding, the powder must also with suitable plasticizers and other additives, such as. B. the functional additives for thermal, weather, UV and IR stabilization are mixed. The mixtures can be tailored to the corresponding subsequent processing requirements. The compound, which is finished by melting, is used to produce granules that can be delivered to the plastics processors.
properties
Cellulose acetate with the usual degrees of substitution is soluble in acetone , but the primary acetate is not. Unlike pure cellulose fibers such as cotton , viscose and lyocell , it can be processed using the melt spinning process. It is transparent, flame retardant and easy to color. The fiber has a lobed cross-section (which enables the acetate silk fabric made from it to trap air and promote thermal insulation), thus has longitudinal lines and a silk-like sheen. Cellulose acetate can be thermoplastically formed at 180 to 200 ° C. At temperatures above 85 ° C, the silk-like sheen suffers.
The dry strength of cellulose acetate is 10–16 cN / tex below that of viscose or cupro . The wet strength is about 65-75% of the dry strength. The cellulose acetate fiber can be stretched by approx. 30%. Since the reversible elongation is also relatively high, textiles made of cellulose acetate tend to wrinkle less than z. B. viscose.
The moisture absorption of cellulose acetate is rather low at approx. 6%. The fiber does not swell much and dries quickly. Due to the low moisture absorption, cellulose acetate textiles tend to be electrostatically charged , although the plastic itself has little tendency to be charged.
The fibers burn with a slightly bluish flame and form droplets.
use
Cellulose acetate is mainly processed into textile fibers and fabrics. Textiles made from cellulose acetate fibers look very similar to natural silk ( artificial silk ) and feel almost as soft. They are usually crease-resistant and easy to care for. Because of its low swelling and water absorption, it is suitable for raincoat and umbrella fabrics. Blouses, shirts, dresses, linings, ties and women's underwear are also made from it.
In ophthalmic optics, cellulose acetate is used for glasses frames, thanks to its thermoplastic properties it can be easily adapted.
Foils made of cellulose acetate - also known here as triacetate or TAC film - are processed in the optical layers of computer flat screens, mobile phone displays and other LC displays . Likewise, in most cases the sheathing of the ends of the shoelaces is made of cellulose acetate. Cellulose acetate is also mainly used for plastic eyeglass frames.
Due to its impact strength, cellulose acetate has played an important role in screwdriver handles in the tool industry for decades, even though other materials such as polypropylene, polyamide and thermoplastic elastomers have been displacing cellulose acetate more and more in recent years.
Cellulose acetate is used for. B. as a dielectric in metal-lacquer capacitors .
Since the 1910s, cellulose triacetate has gradually replaced celluloid as a carrier for film materials, as it is hardly inflammable compared to cellulose nitrate . First, the amateur film materials were launched on CTA, in the formats 9.5 and 16 . Therefore, films with a layer support of acetyl cellulose contributed a few years ago the term Safety film or safety film . Today, the largest use of cellulose acetate fiber is in the manufacture of cigarette filters .
Transparent cellulose acetate cubes
Environmental pollution and degradability

Since around 4.5 billion cigarette filters made of cellulose acetate are currently being released into the environment each year and are therefore suspected of being the most common garbage in the environment, cellulose acetate has a major impact on the environment.
Contrary to what was assumed for a long time, cellulose acetate can in principle be degraded in nature, regardless of the degree of substitution, the rate of degradation being strongly dependent on the degree of substitution: the higher the degree of substitution, the longer the degradation takes. In addition, the rate of degradation strongly depends on the environmental conditions. In highly biologically active soils, CA fibers can, for example, be completely degraded between 4 and 9 months, whereas degradation in other areas can take several years.
In principle, two types of degradation can be distinguished: biological degradation via microorganisms and photodegradation.
Biodegradation takes place in two steps. First, the acetyl groups are broken down by acetyl esterases , so that cellulose is formed. This is then completely broken down by cellulases . One reason for the slower breakdown of CA with a higher degree of substitution is that microorganisms no longer perceive it as a source of food from a degree of substitution greater than 1.
Cellulose acetate absorbs UV light with wavelengths of 280 nm or less. Since the filtered sunlight does not reach wavelengths smaller than 300 nm, no direct noticeable photodegradation is possible. However, indirect photodegradation can be induced in nature by secondary effects or by the addition of photocatalysts such as TiO 2 .
Due to the strong environmental pollution, research is being carried out on numerous approaches that can accelerate the breakdown of cellulose acetate. One example is the installation of an acid depot, which releases acid after smoking a cigarette. The acid can accelerate the first deacetylation degradation step.
maintenance
Due to the chemical modification, cellulose acetate is more resistant to microbial degradation than cellulose. The resistance depends on the degree of substitution of the cellulose fibers. Molds , bacteria and termites break down cellulose acetate into carbon dioxide , water, oxygen and humus . The lightfastness is good, and thanks to its insensitivity to microorganisms, cellulose acetate is also well protected against the effects of the weather .
The fiber is very sensitive both to acids - here in particular inorganic acids such as sulfuric acid - and to alkalis. Compared with oxidizing agents , such as are used in textile finishing, the fiber is stable, but not with respect to the organic solvents in dry cleaning. In particular, care should be taken with stain removers.
Strongly alkaline detergents should not be used due to their sensitivity to lye . The smooth structure of the fiber, and thus the reduced absorption of dirt, make hot laundry unnecessary. In order not to destroy the silk-like shine, most manufacturers recommend ironing cellulose acetate only moderately warm (level 1) in a semi-damp state on the back or inside.
literature
- A. Oak green: acetyl cellulose . (PDF) In: Ullmann's encyclopedia of technical chemistry . Volume 1. 1914, pp. 114-131
- Dissertation 1931 (PDF) on the acetylation of cotton and other cellulose
Web links
- Cellulose acetate at ChemPage.de
- Cellulose acetate . Material archive - extensive material information and pictures
- Generation Zero (PDF; 1.0 MB) Bioplastics Magazine
Individual evidence
- ↑ Wilbrand Woebeken (Ed.): Kunststoff-Lexikon . Carl Hanser Verlag, Munich / Vienna 1998, ISBN 3-446-17969-0 , p. 78.
- ^ Karl Oberbach (Ed.): Saechtling plastic pocket book. Carl Hanser Verlag, Munich / Vienna 2004, ISBN 3-446-22670-2 , p. 551.
- ↑ Wolfgang Kaiser : Synthetic chemistry for engineers - From synthesis to application. 3. Edition. Carl Hanser Verlag, Munich 2011, ISBN 978-3-446-43047-1 , p. 346.
- ↑ Jürgen Falbe, Manfred Regitz (Ed.): Römpp - Lexikon Chemie. Volume 1, 10th, completely revised. Edition. Georg Thieme Verlag, Stuttgart 1996, ISBN 3-13-107830-8 , p. 638.
- ↑ Hans Beyer: Textbook of organic chemistry . S. Hirzel Verlag, Leipzig 1968, p. 341.
- ^ Edward Chauncey Worden: Technology of Cellulose Esters. Volume Eight: Carbohydrate Carboxylates (Cellulose Acetate) . D. Van Nostrand Company, New York 1916, p. 2530.
- ↑ Victor Emmanuel Yarsley: About the production and physical properties of cellulose acetates. Julius Springer Verlagbuchhandlung, Berlin 1927, p. 5.
- ^ A b Stefan Mecheels, Herbert Vogler, Josef Kurz: Culture and industrial history of textiles . Wachter, Bönnigheim 2009, ISBN 978-3-9812485-3-1 , p. 417.
- ↑ Patent GB9.676, filed May 17, 1894
- ^ Charles E. Mullin: Acetate Silk and its Dyes . D. Van Nostrand Company, New York 1927, pp. 18 f.
- ^ Edward Chauncey Worden: Technology of Cellulose Esters. Volume Eight: Carbohydrate Carboxylates (Cellulose Acetate) . D. Van Nostrand Company, New York, 1916, p. 2534.
- ↑ Patent DE 105347 (filed on August 26, 1898) (PDF) Commons
- ↑ Patent DE 112817 (filed on November 25, 1898) (PDF) Commons
- ↑ Valentin Hottenroth: The artificial silk. 2nd, expanded edition. Verlag S. Hirzel, Leipzig 1930, p. 325.
- ↑ Patent DE118538 (filed on August 19, 1899) (PDF) Commons . - Patent DE120713 (applied for on August 18, 1900) . - DE163316 (registered on September 4, 1901)
- ^ Edward Chauncey Worden: Technology of Cellulose Esters. Volume 8: Carbohydrate Carboxylates (Cellulose Acetate) . D. Van Nostrand Company, New York, 1916, p. 2536.
- ↑ patent DE159524 filed August 2, 1901
- ↑ Valentin Hottenroth: The artificial silk . 2nd, expanded edition. Verlag S. Hirzel, Leipzig 1930, p. 18.
- ↑ Miles patent US 838350 (filed November 23, 1904)
- ↑ Valentin Hottenroth: The artificial silk . 2nd, expanded edition. Verlag S. Hirzel, Leipzig 1930, p. 19.
- ↑ BASF patent DE184201 filed on October 2, 1904
- ↑ Bayer patent US790565 (triacetyl cellulose) and Bayer patent US734123 (acetyl cellulose) , both filed on January 8, 1902 by Arthur Eichengrün and Theodor Becker .
- ↑ Patent DE252706, filed September 30, 1905
- ^ Charles E. Mullin: Acetate Silk and its Dyes . D. Van Nostrand Company, New York 1927, p. 19.
- ↑ Bayer word mark DE412799 "Cellit" dated June 17, 1929, deleted April 5, 2001. - Note: the trade name Cellit was used by Bayer as early as 1905.
- ↑ Patent GB 28,733, filed December 28, 1904
- ^ Paul-August Koch , Günther Satlow: Large Textile Lexicon: Specialized lexicon for the entire textile industry . Volume A-K. Deutsche Verlags-Anstalt, Stuttgart 1965, p. 19.
- ↑ Elisabeth Vaupel: Laurel for oak green - homage to a forgotten Jewish chemist . In: Culture & Technology. 1/2005, p. 48. deutsches-museum.de (PDF; 8.3 MB)
- ↑ Elisabeth Vaupel: Laurel for oak green - homage to a forgotten Jewish chemist . In: Culture & Technology. 1/2005, p. 49. deutsches-museum.de (PDF; 8.3 MB) accessed on November 6, 2013
- ↑ The historical development of injection molding technology. at: mhborowski.de , accessed on November 6, 2013.
- ^ Charles E. Mullin: Acetate Silk and its Dyes . D. Van Nostrand Company, New York 1927, p. 20.
- ↑ Valentin Hottenroth: The artificial silk. 2nd, expanded edition. Verlag S. Hirzel, Leipzig 1930, p. 19.
- ↑ Hermann Klare: History of chemical fiber research . Akademie-Verlag, Berlin 1985, p. 55.
- ^ Charles E. Mullin: Acetate Silk and its Dyes . D. Van Nostrand Company, New York 1927, p. 21.
- ↑ Edeltraud Hinkelmann: From the guest house to shimmering colors - On the history of a chemical company . In: Berlin monthly magazine ( Luisenstädtischer Bildungsverein ) . Issue 7, 1999, ISSN 0944-5560 , p. 31-32 ( luise-berlin.de ).
- ↑ Stefan Mecheels, Herbert Vogler, Josef Kurz: Culture and industrial history of textiles. Wachter, Bönnigheim 2009, ISBN 978-3-9812485-3-1 , p. 418.
- ↑ Ludwig Bottenbruch (Ed.): Kunststoff-Handbuch 3/1 - Technical thermoplastics: polycarbonates, polyacetates, polyesters, cellulose esters . Carl Hanser Verlag, Munich / Vienna 1992. ISBN 3-446-16368-9 , pp. 404-408
- ↑ Menachem Lewin (Ed.): Handbook of Fiber Chemistry . Third edition. Taylor & Francis Group, Boca Raton 2007, ISBN 0-8247-2565-4 , pp. 778-784.
- ↑ Zakhar Aleksandrovič Rogowin: Man-made fibers: chemistry - technology . Georg Thieme Verlag, Stuttgart / New York 1982, ISBN 3-13-609501-4 , pp. 182-186.
- ↑ Hans Domininghaus (Ed.): The plastics and their properties . 6th, revised and expanded edition, Springer-Verlag, Berlin / Heidelberg 2005, ISBN 3-540-21410-0 , p. 1461
- ↑ Jürgen Thorwald : The hour of the detectives. Becomes and worlds of criminology. Droemer Knaur, Zurich and Munich 1966, p. 480 f.
- ↑ Florian Rötzer: Not plastic, cigarette butts are the most common waste. Retrieved October 8, 2019 .
- ↑ a b c d e f Juergen Puls, Steven A. Wilson & Dirk Hölter: Degradation of Cellulose Acetate-Based Materials: A Review . In: Journal of Polymers and the Environment . tape 19 , 2011, p. 152-165 , doi : 10.1007 / s10924-010-0258-0 .
- ↑ a b c d Raymond M. Robertson, William C. Thomas, Jitendrakumar N. Suthar & David M. Brown: Accelerated degradation of cellulose acetate cigarette filters using controlled release acid catalysis . In: Green Chemistry . tape 14 , 2012, p. 2266-2272 , doi : 10.1039 / c2gc16635f .
- ↑ Gottfried W. Ehrenstein, Sonja Pongratz: Resistance of plastics . Hanser Fachbuch, 2005, ISBN 3-446-21851-3 , pp. 853-854.