Afatinib
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Afatinib | |||||||||||||||||||||
| other names |
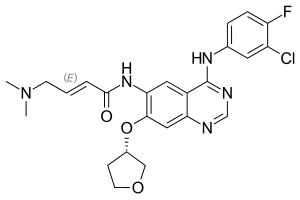
4 - [(3-chloro-4-fluorophenyl) amino] -6 - {[4- ( N , N -dimethylamino) -1-oxo-2-buten-1-yl] amino} -7 - [( S ) tetrahydrofuran-3-yloxy] quinazoline |
|||||||||||||||||||||
| Molecular formula | C 24 H 25 ClFN 5 O 3 | |||||||||||||||||||||
| Brief description |
white to brown solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 485.95 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.38 g cm −3 |
|||||||||||||||||||||
| Melting point |
100-102 ° C |
|||||||||||||||||||||
| pK s value |
11.79 |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Afatinib (approved as Giotrif ; manufacturer: Boehringer Ingelheim ) is an active ingredient from the group of tyrosine - inhibitors , in the treatment of metastatic non-small cell lung cancer is used.
Clinical information
Application areas (indications)
Afatinib is an approved by the FDA in 2013 for the treatment of drug in adult patients with locally advanced and / or metastatic non-small cell lung cancer (NSCLC = non small cell lung carcinoma ) with activating EGFR mutations . A test for EGFR mutations must be positive beforehand . A use in the therapy of breast cancer and squamous cell carcinoma of the head and neck (SCCHN = squamous cell carcinoma of head and neck ) is being investigated.
Type and duration of application
The tablets are taken orally once a day on an empty stomach. Afatinib should not be taken with food; d. H. Food should not be consumed for at least 3 hours before and at least 1 hour after taking this medicine.
Contraindications (contraindications)
Therapy is not recommended in patients with severe impairment of kidney and liver function; dose adjustment may be necessary.
Drug interactions
Simultaneous treatment with strong P-glycoprotein inducers can reduce afatinib plasma levels or increase P-glycoprotein inhibitors.
Use during pregnancy and breastfeeding
The active ingredient is contraindicated during pregnancy and breastfeeding.
Adverse effects (side effects)
The most common side effects that can occur are diarrhea, stomatitis , paronychia , decreased appetite, dysgeusia, and rashes. Increases in the levels of liver enzymes such as ALAT or ASAT can be observed in patients treated with 40 mg afatinib, although these are mostly transient.
Pharmacological properties
Mechanism of action (pharmacodynamics)
Afatinib is a strong and selective inhibitor of the ErbB family of receptor tyrosine kinases , binds as a Michael acceptor via Cys residues of the EGFR to all of the members of the ErbB family EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4 Homo- and heterodimers and irreversibly blocks signal transduction via these receptors.
Absorption and distribution in the body (pharmacokinetics)
Afatinib is 95% bound to human plasma proteins in vitro , both covalently and non-covalently. Elimination occurs via the stool. The plasma half-life is approximately 37 hours. The LogP value is 1.569.
Other Information
Afatinib can impair your ability to react, especially if you also drink alcohol.
Reactivity analysis
For qualitative analysis, the detection of tertiary amines is carried out by alkylation using alkyl halides or for double bonds with the Baeyer sample . Due to the basic tertiary amines, an alkalimetric titration is suitable for quantitative analysis.
FT-IR
FT-IR: (cm −1 ): 3547.4, 2980.2, 2947.8, 2865.7, 2774, 1673.1, 1626.9, 1575.8, 1536.1, 1500.1, 1455.7, 1430.5, 1397.0, 1233.4, 1147.1, 981.9, 852.1, 778.5, 660.9.
Mass spectroscopy
Solvent: methanol. Afatinib can be found at the peak 486 m / z and 488 m / z.
Early benefit assessment
In Germany, since 2011, newly approved drugs with new active ingredients must be subjected to an " early benefit assessment " by the Federal Joint Committee (G-BA) in accordance with Section 35a SGB V if the pharmaceutical manufacturer wants to achieve a higher sales price than just the fixed amount . Only if there is an additional benefit can the pharmaceutical manufacturer negotiate a price with the umbrella association of statutory health insurance companies. The dossier evaluations, on the basis of which the G-BA makes its decisions, are created by the Institute for Quality and Efficiency in Health Care (IQWiG) .
The first G-BA decision of May 8, 2014 was repealed by a further benefit assessment procedure. In 2015, the added benefit of afatinib for the treatment of EGFR-TKI-naive adults with locally advanced and / or metastatic non-small cell lung cancer (NSCLC) with activating EGFR mutations was assessed. According to the G-BA decision, there is an indication of a significant added benefit only for previously untreated patients with ECOG performance status 0 or 1 and the EGFR mutation Del19 compared to the ACT cisplatin plus pemetrexed . An added benefit has not been proven for other EGFR mutations, for those affected with ECOG performance status 2 or after pretreatment with platinum-based chemotherapy. In 2016, afatinib was expanded to treat locally advanced or metastatic NSCLC with squamous cell histology that progresses during or after platinum-based chemotherapy and compared with docetaxel or best supportive care . According to the G-BA decision, an additional benefit for these patients has not been proven.
Individual evidence
- ↑ EU: EMA / 491185/2013, Committee for Medicinal Products for Human Use - Giotrif - Procedure No. EMEA / H / C / 002280 (PDF)
- ↑ entry to afatinib in ChemBlink , accessed on February 2, 2014.
- ↑ Patent WO2007085638 : Process for preparing aminocrotonylamino-substituted quinazoline derivatives. Published on August 2, 2007 , inventors: Jürgen Schröder, Georg Dziewas, Thomas Fachinger, Burkhard Jäger, Carsten Reichel, Svenja Renner.
- ↑ ChEMBL: Compound Report Card
- ↑ a b c eurodiagnostico: Afatinib Datsheet (PDF)
- ↑ a b c Boehringer: Gilotrif MSDS ( Memento from December 27, 2013 in the Internet Archive ).
- ↑ CK Lee, C. Brown, RJ Gralla, V. Hirsh, S. Thongprasert, CM Tsai, EH Tan, JC Ho, da Chu, A. Zaatar, JA Osorio Sanchez, VV Vu, JS Au, A. Inoue, SM Lee, V. Gebski, JC Yang: Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. In: Journal of the National Cancer Institute . Volume 105, Number 9, May 2013, pp. 595-605, doi: 10.1093 / jnci / djt072 . PMID 23594426 .
- ^ BC Liao, CC Lin, JC Yang: First-line management of EGFR-mutated advanced lung adenocarcinoma: recent developments. In: Drugs . Volume 73, Number 4, March 2013, pp. 357-369, doi: 10.1007 / s40265-013-0020-8 . PMID 23479407 .
- ↑ E. Geuna, F. Montemurro, M. Aglietta, G. Valabrega: potential of afatinib in the treatment of patients with HER2-positive breast cancer. In: Breast cancer (Dove Medical Press). Volume 4, 2012, pp. 131-137, doi: 10.2147 / BCTT.S25868 . PMID 24367201 . PMC 3846413 (free full text).
- ↑ M. Agulnik: New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN). In: Medical Oncology . Volume 29, Number 4, December 2012, pp. 2481-2491, doi: 10.1007 / s12032-012-0159-2 . PMID 22252310 . PMC 3466428 (free full text).
- ↑ Red List 2016, entry on Giotrif
- ↑ JC Yang, N. Reguart, J. Barinoff, J. Köhler, M. Uttenreuther-Fischer, U. Stammberger, D. O'Brien, J. Wolf, EE Cohen: Diarrhea associated with afatinib: an oral ErbB family blocker. In: Expert Review of Anticancer Therapy . Volume 13, Number 6, June 2013, pp. 729-736, doi: 10.1586 / era.13.31 . PMID 23506556 .
- ^ ME Lacouture, D. Schadendorf, CY Chu, M. Uttenreuther-Fischer, U. Stammberger, D. O'Brien, A. Hauschild: Dermatologic adverse events associated with afatinib: an oral ErbB family blocker. In: Expert Review of Anticancer Therapy. Volume 13, Number 6, June 2013, pp. 721-728, doi: 10.1586 / era.13.30 . PMID 23506519 .
- ↑ G. Giaccone, Y. Wang: Strategies for overcoming resistance to EGFR family tyrosine kinase inhibitors. In: Cancer treatment reviews. Volume 37, number 6, October 2011, pp. 456-464, doi: 10.1016 / j.ctrv.2011.01.003 . PMID 21367530 . PMC 3139833 (free full text).
- ↑ Patent WO2012121764 : Novel salts and polymorphic forms of Afatanib. Published on September 13, 2012 , Inventors: Ramesh Matioram Gidwani, Channaveerayya Hiremath, Manoj Dalsingar Yadav, Wolfgang Albrecht, Dirk Fischer.
- ↑ Patent US2003149062 : Use of tyrosine kinase inhibitors for the treatment of inflammatory processes. Published on August 7, 2003 , inventors: Birgit Jung, Hubert Püschner.
- ↑ Benefit assessment procedure for the active ingredient Afatinib - decision canceled, accessed on March 23, 2020.
- ↑ A15-17 Afatinib - Benefit assessment according to Section 35a SGB V (dossier assessment); Accessed March 23, 2020.
- ↑ Benefit assessment procedure for the active ingredient Afatinib (reassessment after the deadline: non-small cell lung cancer, EGFR mutation, EGFR-TKI-naive patients); Accessed March 23, 2020.
- ↑ A16-22 Afatinib (new area of application) - Benefit assessment according to Section 35a SGB V; Accessed March 23, 2020.
- ↑ Benefit assessment procedure for the active ingredient Afatinib (new area of application: non-small cell lung carcinoma, squamous epithelial histology); Accessed March 23, 2020.