Prostaglandin E1
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
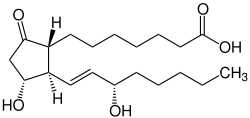
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Alprostadil | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 20 H 34 O 5 | ||||||||||||||||||
| Brief description |
White to pale yellow powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 354.48 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
113-116 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Prostaglandin E 1 is a chemical compound from the group of prostaglandins . It is part of the arachidonic acid - metabolism . Prostaglandin E 1 occurs naturally in animals and is an endogenous substance in humans.
Mechanism of action
Prostaglandin E 1 binds to receptors in the muscle layer of the blood vessels. This allows the vessels to widen. At the same time, the substance has an anticoagulant effect.
In the penis, prostaglandin E 1 causes the muscle cells of the erectile tissue and the cavernous arteries to relax, causing them to widen. Increased blood flows into the erectile tissue, which squeezes the cavernous veins and impedes the outflow of blood. An erection results .
Medical use
Synthetically produced prostaglandin E 1 ( non-proprietary name : Alprostadil ) is used as a medicinal substance for the treatment of various clinical pictures because of its vasodilating ( vasodilating ) and anticoagulant ( platelet aggregation ) effect .
In urology , alprostadil is used as a locally applicable therapeutic agent for erectile dysfunction (ED). The drug can be used for both vascular and neurological or psychological erectile dysfunction in men. The effect sets in within a few minutes and can thus in many cases cause an erection sufficient for sexual intercourse. The risk of systemic side effects is low. There are no known interactions with drugs, food or alcohol. There are no restrictions for cardiac patients who are treated with nitrates or alpha-blockers. Unlike PDE-5 inhibitors , locally administered alprostadil produces an erection even without sexual stimulation.
Alprostadil is available in three dosage forms for the treatment of ED: On the one hand, it can be injected into the penis (cavernous body autoinjection therapy, SKAT) or inserted into the urethra as a stick (medicated urethral system for erection, MUSE). The application is less invasive than cream, which is trickled into the mouth of the urethra. The absorption of alprostadil through the mucous membrane of the urethra is promoted by the permeation enhancer dodecyl ( N , N -dimethyl- DL- alaninate) hydrochloride (DDAIP) contained in the cream . In contrast to SKAT or the use of the MUSE, mechanical damage to the erectile tissue or the urethra can be ruled out with Alprostadil cream.
In the Angiology Alprostadil is used to treat chronic arterial disease used. The main mechanisms are vasodilation (vasodilation) and inhibition of platelet aggregation through activation of the prostaglandin E1 receptor.
In emergency medicine , the active ingredient can be used to keep the ductus arteriosus botalli open in newborns . This measure may be necessary temporarily (for example when transposing the large arteries ) to delay a fatal closure of the ductus botalli until other options to ensure the child's survival (for example balloon septostomy ) are available.
Trade names
For the treatment of erectile dysfunction: Vitaros Creme, Caverject, MUSE.
Individual evidence
- ↑ a b c d e f g h i j k data sheet Prostaglandin E 1 , powder, γ-irradiated, BioXtra, suitable for cell culture at Sigma-Aldrich , accessed on October 10, 2016 ( PDF ).
- ↑ a b c d European Pharmacopoeia, 8th edition, 4th supplement p. 6839 (monograph Alprostadil).
- ↑ a b B. Cuzin: Alprostadil cream in the treatment of erectile dysfunction: clinical evidence and experience . In: Ther Adv Urol . No. 8 (4) , 2016, p. 249-256 , doi : 10.1177 / 1756287216644116 , PMC 5131739 (free full text).
- ↑ V. Hanchanale, I. Eardley: alprostadil for the treatment of impotence . In: Expert Opinion on Pharmacotherapy . No. 15: 3 , 2014, pp. 421-428 , doi : 10.1517 / 14656566.2014.873789 .
- ↑ Uni-due.de: Alprostadil ( Memento from September 26, 2013 in the Internet Archive ) , last viewed on November 27, 2012.

