Altretamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
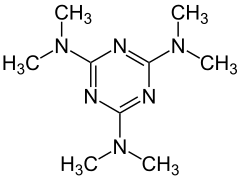
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Altretamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 18 N 6 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 210.28 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
171-175 ° C |
||||||||||||||||||
| solubility |
very bad in water (91 mg l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Altretamine is the generic name of a cytostatic agent [trade name: Hexalen ® (USA)], which chemically is hexamethyl concerns. Altretamine is a six-fold methylated derivative of melamine and is used as a drug in the treatment of ovarian cancer .
Use as a drug and properties
Altretamine is approved for the palliative treatment of patients with relapsed (recurrent) or refractory (non-responsive) ovarian cancer (ovarian cancer) .
The exact mechanism of action of altretamine is still largely unknown. It is assumed that DNA and RNA synthesis in the cell nucleus is inhibited, or that the N -demethylation of altretamine produces reactive intermediates that bind covalently to the DNA. The covalent bond in turn leads to DNA damage .
Altretamine itself is a prodrug that in the liver melamines by oxidative N-demethylation to the actually active N- (hydroxymethyl) metabolizes is. The active ingredient is taken orally, also because of its extremely poor solubility in water . The poor bioavailability when ingested is one of the major disadvantages of the compound.
Altretamine is usually administered in 14 to 21-day cycles with 14 to 21-day breaks between cycles. The main side effects of ingestion are: loss of appetite , nausea and vomiting , and a general feeling of weakness .
Altretamine is weakly mutagenic .
The use of altretamine is more common in the USA than in Germany. In Germany, Austria and Switzerland and also various other European countries, finished medicinal products containing altretamine are not or no longer on the market. Altretamine is designated as a rare disease medicine ( orphan medicine ) in the US .
literature
- DJ Thompson et al: Reproduction and teratology studies on hexamethylmelamine in the rat and rabbit. In: Toxicol Appl Pharmacol 72, 1984, pp. 245-254. PMID 6420936
- C. Forbes et al .: A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer. In: Health Technol Assess 28, 2001, pp. 1-110. PMID 11701100
- BJ Foster: Role of hexamethylmelamine in the treatment of ovarian cancer: where is the needle in the haystack? In: Cancer Treat Rep 70, 1986, pp. 1003-1014. PMID 3089597
- BJ Foster: Hexamethylmelamine: a critical review of an active drug. In: Cancer Treat Rev 13, 1986, pp. 197-217. PMID 3102057
- JK Chan et al .: Oral altretamine used as salvage therapy in recurrent ovarian cancer. In: Gynecol Oncol 92, 2004, pp. 368-371. PMID 14751188
Web links
- Entry on altretamine in the ChemIDplus database of the United States National Library of Medicine (NLM)
Individual evidence
- ↑ a b c d data sheet 2,4,6-tris (dimethylamino) -1,3,5-triazine from Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ↑ Entry on hexamethylmelamine in the SRC PhysProp Database , accessed on May 27, 2009.
- ↑ TC Barnes and S. Frances: Toxicity of hexamethylmelamine (NSC-13875) in rats. In: Arch Int Pharmacodyn Ther 160, 1966, pp. 83-95, PMID 6006448 .
- ^ ES Philips and JB Thiersch: The nitrogen mustard-like actions of 2,4,6-tris (ethylenimino) -s-triazine and other bis (ethylenimines). In: J Pharmacol Exp Ther 100, 1950, pp. 398-407, PMID 14804279 .
- ↑ M. Markman et al .: Altretamine (hexamethylmelamine) in platinum-resistant and platinum-refractory ovarian cancer: a Gynecologic Oncology Group phase II trial. In: Gynecol Oncol 69, 1998, pp. 226-229, PMID 9648592 .
- ↑ TJ Herzog: Recurrent ovarian cancer: how important is it to treat to disease progression? In: Clin Cancer Res 10, 2004, pp. 7439-7449, PMID 15569973 .
- ↑ National Cancer Institute altretamine accessed May 27, 2009.
- ^ HM Coley: N- (Hydroxymethyl) melamines. In: Gen Pharmacol 28, 1997, pp. 177-182, PMID 9013191 .
- ↑ G. Damia and M. D'Incalci: Clinical Pharmacokinetics of altretamine. In: Clinical Pharmacokinetics 28, 1995, pp. 439-448, PMID 7656502 .
- ↑ drugs.com: Altretamine. (accessed September 19, 2012).
- ↑ J. Ashby et al.: Weak mutagenicity to Salmonella of the formaldehyde-releasing anti-tumor agent hexamethylmelamine. In: Mutat Res 142, 1985, pp. 121-125, PMID 3919288 .
- ^ H. Meden: Ovarian carcinoma. Verlag Walter de Gruyter, 1996, ISBN 3-11-014956-7 , p. 60.
- ↑ Pharmaceutical substance list (ABDA database), June 2009.
- ^ List "Orphan Products Designated and / or Approved" on FDA: Designating an Orphan Product: Drugs and Biologics .