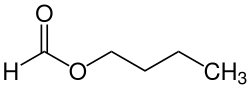
Formic acid n- butyl ester
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Formic acid n- butyl ester | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 10 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid with an alcohol-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 102.13 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.92 g cm −3 |
||||||||||||||||||
| Melting point |
−96 ° C |
||||||||||||||||||
| boiling point |
106 ° C |
||||||||||||||||||
| Vapor pressure |
29 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.389 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Formic acid n- butyl ester is a chemical compound from the group of carboxylic acid esters .
Occurrence
Formic acid n- butyl ester was detected in fresh apples , strawberries , cloudberries , sherry and parmesan .
Extraction and presentation
Formic acid n- butyl ester can be obtained by azeotropic distillation of formic acid and n- butyl alcohol with isopropyl formate or by boiling n- butyl alcohol and formamide in the presence of zinc chloride , zinc sulfate or mercury (II) chloride .
properties
Formic acid n- butyl ester is a highly flammable, volatile, colorless liquid with an alcohol-like odor that hydrolyzes in water. In a lower concentration, the compound has a fruity, plum-like odor and taste.
use
Formic acid n- butyl ester is used as a flavoring substance. The compound is also being investigated as a potential biofuel. It is also rarely used as a solvent for fats, oils, cellulose nitrate , some cellulose ethers and cellulose esters , and also for many natural and synthetic binders.
safety instructions
The vapors of formic acid n- butyl ester can form an explosive mixture with air ( flash point 18 ° C, ignition temperature 265 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on n-butyl formate in the GESTIS substance database of the IFA , accessed on December 11, 2018(JavaScript required) .
- ↑ a b c d George A. Burdock: Fenaroli's Handbook of Flavor Ingredients . CRC Press, 2016, ISBN 978-1-4200-9086-4 , pp. 205 ( limited preview in Google Book search).
- ↑ Data sheet Butyl formats, ≥97%, FG at Sigma-Aldrich , accessed on December 11, 2018 ( PDF ).
- ↑ Entry on butyl formate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 30, 2018. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ EU: IMPLEMENTING REGULATION (EU) No. 872/2012 OF THE COMMISSION of October 1, 2012 laying down the list of flavoring substances according to Regulation (EC) No. 2232/96 of the European Parliament and of the Council, including this list in Annex I. of Regulation (EC) No. 1334/2008 of the European Parliament and of the Council as well as repealing Regulation (EC) No. 1565/2000 of the Commission and Decision 1999/217 / EC of the Commission , accessed on December 11, 2018
- ↑ Stijn Vranckx, Joachim Beeckmann u. a .: An experimental and kinetic modeling study of n-butyl formate combustion. In: Combustion and Flame. 160, 2013, p. 2680, doi : 10.1016 / j.combustflame.2013.06.012 .
- ↑ Entry on butyl formate. In: Römpp Online . Georg Thieme Verlag, accessed on December 11, 2018.

