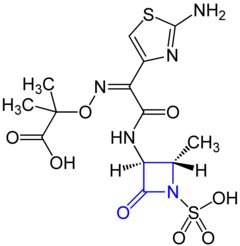
Aztreonam
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Aztreonam | |||||||||||||||||||||
| other names |
( Z ) -2 - [({(2-Amino-4-thiazolyl) [(2 S , 3 S ) -2-methyl-4-oxo-1-sulfo-3-azetidinyl] carbamoyl} methylenamino) oxy] - 2-methylpropionic acid |
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
colorless, odorless, crystalline powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 435.43 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
227 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Aztreonam is a drug selected from the group of the monobactam - antibiotics , in the treatment of infections with gram-negative , aerobic bacteria is used.
application areas
Infections of the kidneys, the lower urinary tract, the lower respiratory tract (including pneumonia and bronchitis), also with cystic fibrosis (as a lysinate inhalation solution Cayston ), meningitis, infections of the bones and joints, of skin and soft tissue, of the abdominal cavity, in gynecology, Aztreonam is used for sepsis and gonorrhea.
Adverse effects (side effects)
Occasionally gastro-intestinal symptoms, rarely headache, dizziness, confusion, changes in the blood count, coagulation disorders and kidney dysfunction, very rarely pseudomembranous colitis, jaundice and tinnitus.
Contraindications and warnings
The contraindication is hypersensitivity to aztreonam. Attention should be paid to the risk of cross-allergy in ceftazidime allergy caused by identical side chains. In addition, it must be used carefully if the patient is allergic to reaction.
Chemical structure
Aztreonam contains - like penicillins and cephalosporins - a β- lactam ring . This is, however - unlike the penicillins and cephalosporins - not to a five- or six-membered heterocyclic ring fused .
Trade names
- Monopreparations : Azactam (A, CH, D), Cayston (A, D)
Web links
- Entries in the NIH study registry
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Aztreonam
Individual evidence
- ↑ a b c d e f The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 156, ISBN 978-0-911910-00-1 .
- ↑ a b Data sheet Aztreonam, ≥98% (HPLC) from Sigma-Aldrich , accessed on December 1, 2019 ( PDF ).
- ↑ Summary of the European Public Assessment Report (EPAR) for Cayston (PDF; 131 kB) published by the European Medicines Agency . P. 3. Retrieved August 30, 2010.
- ↑ ROTE LISTE 2008 , Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-939192-20-6 .
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , p. 339.
- ↑ Marianne Abele-Horn (2009), p. 339.