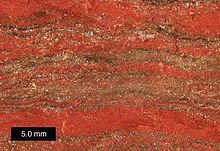
Ribbon ore

A band ore is an iron-bearing, marine sedimentary rock that was mainly deposited in the Precambrian and that has a characteristic layer structure due to metal-bearing layers. In the section perpendicular to the stratification, the strata, consisting primarily of iron minerals, appear as a band structure, to which the ore owes its name in German and English ( Banded Iron Formation , abbreviated BIF).
origin
Ribbon ores originated in the Archean (3800 to 2500 million years ago) and in the Proterozoic (2500 to 1800 million years ago). The iron released into the sea in the course of submarine volcanism and possibly the iron that got into the free weathering cycle of the earth due to various causes remained dissolved in the sea water in the form of divalent ions before this time . It was not immediately oxidized to trivalent iron by the free oxygen (O 2 , Dioxygen) in the sea and atmosphere , which would have been excreted ( precipitated ) in the form of compounds that were difficult to dissolve in water . Trivalent iron was not formed due to the lack of O 2 in the sea water and the atmosphere. Instead, divalent iron accumulated until it was bound by various processes and collected in thin layers on the sea floor. About 3800 million years ago, oxygen phototrophic microorganisms , probably the ancestors of today's cyanobacteria , developed a form of photosynthesis in which O 2 was formed as a waste product (so-called oxygenic photosynthesis). From that time on, the divalent iron was oxidized to trivalent and precipitated in the form of hydroxides and oxides which were deposited in layers. This process consumed the oxygen that was microbially produced in large quantities. The process ran cyclically over a period of several 100 million years, during which the microbially formed free oxygen was always completely bound by the oxidation of the dissolved iron present. The causes of the cyclical course of iron precipitation are not known. Ribbon iron ores were formed from the deposits by diagenesis .
Only when the divalent iron was exhausted and the formation of ligaments was therefore complete, the oxygen content in seawater and in the earth's atmosphere could rise to values above 0.2 to 0.5 percent. This is called the great oxygen catastrophe.
To a limited extent, ribbon ores were still formed in the Cryogenium (750 to 600 million years ago) and in the Ordovician (around 450 million years ago).
construction
Banded iron formation have a layered structure, said iron-containing layers with chert layers (engl. Chert , micro-cryptocrystalline quartz <30 micron particle size) alternate. The main minerals occurring in the ferrous layers are hematite (Fe 2 O 3 ) and magnetite (Fe 3 O 4 ). The individual layers are a few millimeters to a few centimeters thick and give the rock cross-section the appearance of the eponymous banding. They can occur in multiple repetitions, so that ribbon ore formations can have thicknesses (layer thicknesses) of around 50 m to 600 m, making them economically important iron ore deposits .
Types
There are three types:
- the Algoma type, which is lens-shaped and interlocked with volcanic rocks and greywacke (deposits, for example, in Canada and Australia). The volcanic activity was submarine.
- the superior type, which has a larger area due to its origin in shelf areas . A relationship to volcanic activity is not obvious with this type.
- the Rapitan type, which occurs at the end of the Neoproterozoic in connection with glacial sediments (e.g. Tillites ) ( snowball earth ).
Emergence

The origin of the band ores has been intensively investigated, but it has not yet been conclusively clarified. One of the main points of contention is the role of bacteria in the formation of the band ores and the development of the oxygen concentration over time in connection with the question of whether the oxygen was present in sufficiently high concentrations in the atmosphere at the time when the band ores were formed.
It is generally assumed that the iron was of volcanic origin and was added to the sea water through exhalation on the mid-ocean ridges and along deep-sea trenches . The investigation of the distribution and the content of rare earths revealed an abnormally high content of europium in the rock, which confirms the volcanic origin, as well as the Nd isotopic composition of the chert layers. Against the thesis that the iron comes from the weathering of continental rocks, the low content of aluminum in some BIFs and the fact that mostly no clastic sediments such as clays occur together with the ribbon ores speak against them.
One explanation for the formation of the BIFs is an interplay between biological activity and iron that has entered the upper water layers and shallow water through vertical currents. Due to the oxygen production from the oxygenic photosynthesis of the cyanobacteria that existed at that time , the iron dissolved in the seawater was oxidized immediately and hard water-soluble hydroxidic and oxidic compounds of trivalent iron (including iron (III) hydroxide and iron (III) oxyhydrates) were formed ). These poorly water-soluble minerals sedimented and the minerals magnetite and hematite were formed during diagenesis through the release of water . It is assumed that the absorption of oxygen by the iron dissolved in sea water only lasted a certain time, namely until the available iron was used up and the released oxygen was no longer bound by iron. This is said to have resulted in an oxygen concentration that is harmful to the cyanobacteria, which led to the death of the bacteria. Subsequently the chert sedimented. These appear to have been formed by direct abiotic precipitation of silicon dioxide and by organisms that precipitate silicon dioxide .
Another possibility for the oxidation of divalent iron is the activity of anoxygenic phototrophic bacteria, which use light as an energy source to produce biomass from carbon dioxide and water by using divalent iron as a reducing agent and thereby oxidizing it to trivalent iron, but no oxygen (dioxygen, O 2 ) form. Model calculations have shown that a relatively thin layer of such anoxygenic phototrophic bacteria is sufficient to oxidize and thus precipitate all dissolved iron in seawater.
Abiotic formation is also believed to be possible: Ions of divalent iron are oxidized to trivalent iron ions by UV radiation and blue light up to a wavelength of around 400 nm, with the electrons being transferred to hydrogen ions and thus molecular, elementary hydrogen (H 2 ) is formed: 2 Fe 2+ + 2 H + → 2 Fe 3+ + H 2 . The trivalent iron ions form hematite together with water or magnetite together with divalent iron ions and water.
The origin of the band ores appearing after a long time at the exit of the Proterozoic is also not fully understood. On the one hand, they are seen as evidence for the Snowball Earth hypothesis: With the high oxygen concentrations in the sea and atmosphere at that time, the complete ice cover of the oceans is the condition for the sea water to become anoxic and to be able to absorb dissolved iron in large quantities. The iron is oxidized as the ice melts and separates out as sediment. On the other hand, they are explained as the formation of metal-rich, anoxic sea waters. Their metal content is of volcanic origin, their place of formation are rift basins of tectonic origin, whose water layers close to the ground are often anoxic. For the disappearance of the banded iron ores around 1.8 billion years ago, a better mixing of the ocean with oxygen is assumed, possibly in the wake of the Sudbury impact .
Occurrence
According to the Proterozoic Age, the regional distribution of the ribbon ores is tied to the shield areas of the continents in which rocks of this age occur. Strip ores are of enormous economic importance, and a considerable proportion is already exploitable today. Their iron reserves are estimated at 150 billion tons, which speaks for a long range of iron reserves with a consumption of around one billion tons per year.
Large deposits with an age of 2.6-2.1 Ga (billion years) are located in the Transvaal, South Africa. In Australia, there are large reserves in the 2.7–2.4 Ga old strip ores of the Hamersley Range. In Krivoy Rog , Ukraine, the band ores are 2.6–1.9 Ga old, and those in the state of Minas Gerais ( Itabirite ), Brazil, and in the Labrador trough in Canada are about the same . Some occurrences were later metamorphically influenced one or more times by tectonic or regional metamorphic processes.
In addition to iron, other metals also occur to a lesser extent in the strip ores, which also have a certain economic importance.
Trimmed banded iron formation of the Dales Gorge Formation near Pannawonica, Pilbara, Western Australia
literature
- Anthony M. Evans: Mineral Deposition Studies. Pp. 90f., 257ff., 279, 316f. Ferdinand Enke Verlag, Stuttgart 1992, ISBN 3-432-99801-5 .
- Bekker, A., Slack, JF, Planavsky, N., Krapež, B., Hofmann, A., Konhauser, KO, and Rouxel, OJ, 2010, Iron formation: The sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes , Economic Geology, v. 105, 467-508. PDF
Individual evidence
- ^ A b Paul F. Hoffman, Daniel P. Schrag: The snowball Earth hypothesis: testing the limits of global change (PDF; 1.3 MB). Terra Nova 14, pp. 129-155, 2002.
- ↑ http://www.mineralienatlas.de/lexikon/index.php/RockData?rock=B%E4ndererz
- ^ A b Andreas Kappler, Claudia Pasquero, Kurt O. Konhauser, Dianne K. Newman: Deposition of banded iron formations by anoxygenic phototrophic Fe (II) -oxidizing bacteria , In: Geology ; Vol. 33, No. 11, 2005, pp. 865–868 ( PDF )
- ↑ Cornelis Klein: Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origins . In: American Mineralogist . tape 90 , no. 10 , 2005, pp. 1473-1499 , doi : 10.2138 / am 2005.1871 .
- ↑ Horstmann, UE et al .: Rare earth elements and Nd isotopic compositions in banded iron formations of the Griqualand West Sequence, Northern Cape Province, South Africa. Journal of the German Society for Geosciences, Volume 152, Issue 2-4, pp. 439-465. Abstract
- ↑ a b Mike Strickler: Banded Iron Formation. University of Oregon (English, HTML ).
- ↑ F. Widdel, S. Schnell, S. Heising, A. Ehrenreich, B. Assmus, B. Schink: Ferrous iron oxidation by anoxygenic phototrophic bacteria . In: Nature Vol. 362, 1993, pp. 834-836
- ↑ Armin Ehrenreich, Friedrich Widdel: Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism . In: Applied and Environmental Microbiology. Vol. 60, No. 12, 1994, pp. 4517-4526.
- ↑ Christian de Duve: Origin of Life - Prebiotic Evolution and the Origin of the Cell . Spectrum Academic Publishing House, Heidelberg / Berlin / Oxford 1994, ISBN 3-86025-187-2 , pp. 142-143.
- ↑ N. Eyles, N. Januszczak: 'Zipper rift': A tectonic model for Neoproterozoic glaciations during the breakup of Rodinia after 750 Ma . In: Earth Science Reviews . Vol. 65, No. 1–2, 2004, pp. 1–73 doi : 10.1016 / S0012-8252 (03) 00080-1 ( PDF, 4 MB ( Memento of the original from November 28, 2007 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. ).
- ↑ John F. Slack, Cannon, William F .: Extraterrestrial demise of banded iron formations 1.85 billion years ago . In: Geology . 37, No. 11, 2009, pp. 1011-1014. doi : 10.1130 / G30259A.1 .
- ^ Very Large Fe-Deposits ( Memento of November 23, 2008 in the Internet Archive ), Columbia University in the City of New York, Lamont-Doherty Earth Observatory
Web links
- Bacteria in the primordial ocean stored iron ore from the University of Tübingen (ed.), September 8, 2008