Benzyldimethylamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
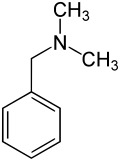
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Benzyldimethylamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 13 N | |||||||||||||||
| Brief description |
flammable, colorless liquid with an amine-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 135.21 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.90 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−75 ° C |
|||||||||||||||
| boiling point |
181 ° C |
|||||||||||||||
| Vapor pressure |
2.4 mbar (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.501 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Benzyldimethylamine is a chemical compound from the group of amino compounds .
Extraction and presentation
Benzyldimethylamine can be obtained by reacting benzylamine with methanol in the presence of hydrogen chloride as a catalyst .
It is also possible to prepare it by reacting benzyl chloride with dimethylamine .
properties
Benzyldimethylamine is a flammable, less volatile, colorless liquid with a foul-smelling amine-like odor, which is sparingly soluble in water. Its aqueous solution has an alkaline reaction. It has a viscosity of 3 mPa · s at 20 ° C.
use
Benzyldimethylamine is used in the manufacture of polyurethane paints, coatings, foams and potting compounds and as an intermediate in organic syntheses. It is also used in electron microscopy as a so-called Maraglass catalyst.
safety instructions
The vapors of benzyldimethylamine can form an explosive mixture with air ( flash point 55 ° C, ignition temperature 410 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on benzyldimethylamine in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b Data sheet N , N -Benzyldimethylamine (PDF; 223 kB) from GisChem, accessed on August 13, 2012.
- ↑ Data sheet N, N-Dimethylbenzylamine from Sigma-Aldrich , accessed on September 30, 2010 ( PDF ).
- ↑ Entry on Benzyldimethylamine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Dilip K. Nandi, Sunanda K. Palit, Dibakar C. Deka: Acid catalyzed pressure synthesis of N, N-dimethylbenzylamine from benzylamine and methanol. In: Journal of Chemical Technology & Biotechnology. 38, 1987, p. 243, doi : 10.1002 / jctb.280380404 .
- ↑ Data sheet N, N-Dimethylbenzylamine (PDF) from Merck , accessed on September 30, 2010.
- ↑ Konrad Uhlig: Polyurethane Pocket Book , Carl Hanser Verlag Munich, Vienna, 1998, p. 130, ISBN 3-446-18913-0 .


