Brazilian
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
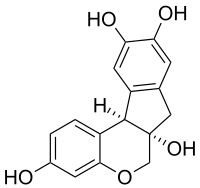
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Brazilian | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 14 O 5 | ||||||||||||||||||
| Brief description |
pale yellow needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 286.28 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
250 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Brazilin is a natural dye . It was first isolated from brazil wood in 1808 by the French chemist Eugène Chevreul . Due to its chemical structure, Brazil is classified in the neoflavonoids group.
properties
Brazilin forms pale yellow needles and shows fluorescence .
Recent studies indicate a pharmacological effectiveness of Brazil as an antagonist of the collagen receptor. Brazilin has a toxic effect on some tumor cells in cell culture by inducing apoptosis . Brasilin has an antibacterial effect.
Brasilin can be obtained from crushed redwoods by extraction with methanol . In addition, various synthetic routes of Brazil have been described. Compared to the related dye hematoxylin, Brazilin lacks a hydroxyl group. Alkaloid analogues can be produced from Brazil .
use

Braziline was previously used as a dye and, along with its oxidation product, Braziline, is the main coloring component of many types of redwood, the heartwood of which was previously used in wool and cotton dyeing. When storing or Zerraspeln of the harvested timber brasilin oxidized slowly to brasilein. In Nuremberg , the profession of Brazil wood chopper is documented for the 16th century. Today mainly bows for string instruments are made from the colored wood.
Together with the deep red brazil it also serves as an acid-base indicator . In histology , Brazilin is used to stain cell nuclei in nerve tissues. In painting, Brazilian was used as a red color with a pickling salt (also called mordant ), which makes it a difficult-to-dissolve dye complex.
Individual evidence
- ↑ a b c d e entry on Brazil. In: Römpp Online . Georg Thieme Verlag, accessed on June 23, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Luiz FC De Oliveira, Howell GM Edwards, Eudes S. Velozo, M. Nesbitt: Vibrational spectroscopic study of Brazilin and brazilein, the main constituents' of brazil wood from Brazil . In: Vibrational Spectroscopy . 28, No. 2, 2002, p. 243. doi : 10.1016 / S0924-2031 (01) 00138-2 .
- ↑ R. Rondão, JS Seixas de Melo, J. Pina, MJ Melo, T. Vitorino, AJ Parola: Brazil Wood reds: the (photo) chemistry of Brazilin and brazilein. In: The journal of physical chemistry. A. Volume 117, number 41, October 2013, pp. 10650-10660, doi : 10.1021 / jp404789f . PMID 24050687 .
- ↑ Y. Chang, SK Huang, WJ Lu, CL Chung, WL Chen, SH Lu, KH Lin, JR Sheu: Brazilin isolated from Caesalpinia sappan L. acts as a novel collagen receptor agonist in human platelets. In: Journal of biomedical science. Volume 20, 2013, p. 4, doi : 10.1186 / 1423-0127-20-4 . PMID 23350663 . PMC 3564834 (free full text).
- ↑ DY Lee, MK Lee, GS Kim, HJ Noh, MH Lee: Brazilin inhibits growth and induces apoptosis in human glioblastoma cells. In: Molecules. Volume 18, number 2, 2013, pp. 2449-2457, doi : 10.3390 / molecules18022449 . PMID 23429418 .
- ↑ HX Xu, SF Lee: The antibacterial principle of Caesalpinia sappan. In: Phytotherapy research: PTR. Volume 18, number 8, 2004, pp. 647-651, doi : 10.1002 / ptr.1524 . PMID 15476302 .
- ↑ Y. Lu, H. Bai, C. Kong, H. Zhong, MC Breadmore: Analysis of brazilin and protosappanin B in sappan lignum by capillary zone electrophoresis with acid barrage stacking. In: Electrophoresis . Volume 34, number 24, December 2013, pp. 3326–3332, doi : 10.1002 / elps.201300402 . PMID 24150968 .
- ↑ X. Wang, H. Zhang, X. Yang, J. Zhao, C. Pan: Enantioselective total synthesis of (+) - brazilin, (-) - brazilein and (+) - brazilide A. In: Chemical communications. Volume 49, Number 47, June 2013, pp. 5405-5407, doi : 10.1039 / c3cc42385a . PMID 23657435 .
- ^ Y. Huang, J. Zhang, TR Pettus: Synthesis of (+/-) - brazilin using IBX. In: Organic letters. Volume 7, Number 26, December 2005, pp. 5841-5844, doi : 10.1021 / ol0523749 . PMID 16354080 . PMC 2423948 (free full text).
- ^ P. Pfeiffer, J. Breitbach, W. Scholl: Alkaloid-like compounds from Brasilin and Hematoxylin. In: Journal for Practical Chemistry. Volume 154, 1940, pp. 157-208, doi : 10.1002 / prac.19401540601 .
- ↑ Sabine Struckmeier: Textile dyeing from the late Middle Ages to the early modern period (14th-16th centuries): A scientific-technical analysis of German-language sources . Waxmann Verlag, 2011, ISBN 978-3-8309-7527-4 , p. 185 ( limited preview in Google Book search).
- ↑ Entries on Caesalpinia , in the useful plants database of the University of Marburg , accessed on April 2, 2018.
- ↑ Entry Brasilin in the Lexicon of Biology on Spektrum.de
- ^ V. Augulis, J. Sepinwall: Brazilin-toluidine blue O and hematoxylin-darrow red methods for brain and spinal cord. In: Stain technology. Volume 44, Number 3, May 1969, pp. 131-137, PMID 4181667 .