Buflomedil
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
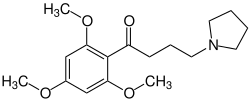
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Buflomedil | ||||||||||||||||||
| other names |
2 ′, 4 ′, 6′-trimethoxy-4- (1-pyrrolidinyl) butyrophenone ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 17 H 25 NO 4 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 307.39 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
192-193 ° C (HCl) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Buflomedil is a drug used to support the treatment of peripheral arterial occlusive disease (PAVK). With this, for example, pain occurs in the legs when walking, as the arteries are narrowed by deposits in their lumen and thus less blood with nutrients reaches the muscle tissue of the legs. The active ingredient promotes blood flow in medium-sized and small arterial blood vessels. It is an unselective antagonist of α- adrenoreceptors , the vasodilatory effect mainly due to α 1 -blockade.
At the beginning of 2012, all drugs containing buflomedil had to be withdrawn from the market in the EU member states because severe side effects had occurred and the therapeutic benefit was assessed as insufficient.
Pharmacological properties
The α 1 -adrenoreceptors are mainly located on the medium-sized and small arteries, for example in the arms and legs. When a chemical binds to this receptor (agonist), it triggers a contraction of the arterial muscles and the diameter of the artery becomes smaller ( vasoconstriction ). As a result, the blood flow in this artery decreases, and with it the blood flow to the tissue that is supplied with blood by this artery. Buflomedil is an antagonist of the α-adrenergic receptors, it binds to the receptor without causing a reaction in the artery. As a result, agonists of the α 1 receptor, which are normally constantly present in small amounts in the blood, can no longer bind to the receptor, since the binding site is already occupied by the antagonist buflomedil and is therefore blocked. The tension of the arterial musculature (muscle tone) decreases and the diameter of the artery increases ( vasodilation ), since in total fewer receptors are occupied by α 1 agonists. The enlargement of the lumen of the artery increases the blood flow and circulation, which is used in peripheral arterial occlusive disease (PAVK).
Side effects
Since buflomedil not only blocks α 1 but to a certain extent also α 2 receptors and these two receptors occur on many other cells (e.g. nerve cells), the safe dosage must be determined very precisely. Accidental overdoses or deliberate violations of the concentration limit of the drug can lead to pronounced side effects with fatal outcome. Buflomedil has a narrow therapeutic index ; H. Dose range in which there is an acceptable relationship between desired and desired effects.
Side effects of the central and peripheral nervous system
The occupation of α 2 receptors in the central nervous system (CNS) generally leads to the inhibition of the activity of nerve cells, and thus generally to the attenuation of the excitation and excitability of the CNS. If these α 2 receptors are blocked, this inhibition disappears and there are side effects that can be explained by the general disinhibition in the CNS. These are mainly focal seizures and generalized tonic-clonic seizures ("grand mal") with unconsciousness, which can progress to a life-threatening event, the status epilepticus, which can lead to death.
These inhibitory α 2 receptors are also found on nerve cells in the periphery outside the central nervous system . Side effects that can be derived here by the blockade of α 2 receptors are z. B. Signs of general overexcitation of nerve cells that coordinate the contraction of the muscles (motor neurons ). As a side effect, muscle cramps ( myoclonia ) can occur here. Muscle spasms can also be the result of disinhibition in the central nervous system due to disinhibition of neurons that are responsible for motor skills.
Side effects of the cardiovascular system (cardiovascular system)
The blockade of α 1 receptors on the blood vessels generally leads to vasodilation ( vasodilation ), as a result of which blood pressure drops sharply (hypotension). This can lead to orthostasis ("circulatory collapse") with brief loss of consciousness. In total, less blood volume also comes back to the heart (preload decreases) and the heart can therefore pump less volume into the circulation per heartbeat. Since the blood vessels themselves supply the heart with less blood (reduced blood flow, ischemia ), damage to the heart muscle tissue can result, including (predominantly ventricular ) cardiac arrhythmias, which in extreme cases can lead to death.
Withdrawal from the market
Buflomedil was marketed in Germany as a prescription drug from 1982 under the trade name Bufedil . The use of buflomedil was discussed because of the severe side effects caused by the narrow therapeutic range. In France, oral preparations containing buflomedil (but not the infusion) were withdrawn from the market in 2006. In May 2011, the European Medicines Agency (EMA) recommended that the approval for oral buflomedil-containing drugs be suspended in all member states until the risk-benefit ratio had been finally clarified. Several German pharmaceutical companies then voluntarily withdrew buflomedil from the market. In November 2011, the EMA extended the recommendation to include parenteral dosage forms. The limited benefits of buflomedil do not outweigh the risk of serious cardiac and neurological side effects. In February 2012, the European Commission issued the relevant implementation decision to all member states.
literature
- Wolfgang Forth, Dietrich Henschler and Walter Rummel: General Pharmacology & Toxicology. ; Urban & Fischer Verlag; 8th edition 2001 p. 191 “Chapter 4 - Pharmacology of noradrenergic and adrenergic systems: 4.5 α-adrenoceptor antagonists”; ISBN 3-437-42520-X
- Andreas Ruß (ed.) And Stefan Endres (ed.): Arzneimittel Pocket plus 2008 , Börm Bruckmeier, 4th edition 2007 p. 68, p. 87; ISBN 3-89862-287-8
- ifap database Buflomedil
- M. Boeckh, T.Böckers: "GK2 General Pharmacology and Toxicology"; Thieme Verlag; 15th edition 2002 p. 179 "Chapter 3 - Interventions in the sympathetic nervous system" ISBN 3-13-112535-7 .
Individual evidence
- ↑ a b Datasheet BUFLOMEDIL HYDROCHLORIDE CRS (PDF) at EDQM , accessed on August 11, 2008.
- ↑ Buflomedil hydrochloride data sheet from Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
- ↑ Agence nationale de sécurité du médicament et des produits de santé (ANSM): Pharmacovigilance et la sécurité d'emploi du buflomédil. Released November 30, 2006.
- ↑ European Medicines Agency (EMEA): European Medicines Agency recommends suspension of oral buflomedil-containing medicines published May 20, 2011.
- ↑ EMA press release of November 17, 2011, available as a pdf, last accessed on November 19, 2011.
- ↑ Implementation decision of the European Commission of February 13, 2012, available as a pdf , accessed on July 22, 2018.