Cannabinol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
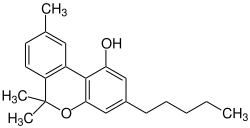
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cannabinol | |||||||||||||||
| other names |
6,6,9-trimethyl-3-pentyl-6 H -dibenzo [ b , d ] pyran-1-ol ( IUPAC ) |
|||||||||||||||
| Molecular formula | C 21 H 26 O 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 310.43 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
77 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cannabino l ( CBN ) is a slightly psychoactive cannabinoid that is only found in traces in cannabis and is mainly found in aged cannabis . Pharmacologically relevant quantities are formed as a metabolite of tetrahydrocannabinol ( THC ). CBN acts as a partial agonist on the CB1 receptors , but has a higher affinity for CB2 receptors ; however, it has lower affinities compared to THC . Degraded or oxidized cannabis products such as traditionally made hashish are high in CBN.
When cannabis is exposed to air or ultraviolet light (e.g. sunlight ) for a long period of time , THCA converts to cannabinolic acid (CBNA). CBN is then formed by decarboxylation of CBNA.
chemistry
Chemically speaking, cannabinol (CBN) and cannabidiol (CBD) are similar. In contrast to THC, CBN has neither double bond isomers nor stereoisomers .
legality
CBN is not listed in the United Nations Standard Convention on Narcotic Drugs of 1961 , nor in the Convention on Psychotropic Substances of 1971, so CBN is largely classified as legal. There are marketability restrictions in Canada.
Individual evidence
- ↑ a b Entry on cannabinol in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 19, 2016.
- ↑ Data sheet Cannabinol solution, 1.0 mg / mL in methanol, analytical standard, for drug analysis from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ Biotrend: Cannabinol ( Memento of the original from May 22, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF file; 21 kB)
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics . CRC Press, 2012, ISBN 1-4398-8049-2 , pp. 3–90 ( limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Karniol IG: Effects of Δ9-Tetrahydrocannabinol and Cannabinol in Man. In: Krager. Retrieved March 12, 2020 .
- ↑ Anu Mahadevan, Craig Siegel, Billy R. Martin, Mary E. Abood, Irina Beletskaya: Novel Cannabinol Probes for CB1 and CB2 Cannabinoid Receptors . In: Journal of Medicinal Chemistry . tape 43 , no. October 20 , 2000, ISSN 0022-2623 , p. 3778-3785 , doi : 10.1021 / jm0001572 ( acs.org [accessed March 12, 2020]).
- ↑ NK McCallum, B. Yagen, S. Levy: Cannabinol: a rapidly formed metabolite of 520-1520-1520-1 . In: Experientia . tape 31 , no. 5 , May 1975, ISSN 0014-4754 , pp. 520-521 , doi : 10.1007 / BF01932433 ( springer.com [accessed March 12, 2020]).
- ↑ Anu Mahadevan, Craig Siegel, Billy R. Martin, Mary E. Abood, Irina Beletskaya: Novel Cannabinol Probes for CB1 and CB2 Cannabinoid Receptors . In: Journal of Medicinal Chemistry . tape 43 , no. October 20 , 2000, ISSN 0022-2623 , p. 3778-3785 , doi : 10.1021 / jm0001572 ( acs.org [accessed March 12, 2020]).
- ↑ François Petitet, Bernadette Jeantaud, Michel Reibaud, Assunta Imperato, Marie-Christine Dubroeucq: Complex pharmacology of natural cannabivoids: Evidence for partial agonist activity of Δ9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors . In: Life Sciences . tape 63 , no. 1 , May 1998, pp. PL1 – PL6 , doi : 10.1016 / S0024-3205 (98) 00238-0 ( elsevier.com [accessed March 12, 2020]).
- ↑ Cannabinol (code C84510). Retrieved March 12, 2020 .
- ^ Conventions. Retrieved March 25, 2019 .
- ^ Legislative Services Branch: Consolidated federal laws of Canada, Controlled Drugs and Substances Act. October 17, 2018, accessed March 25, 2019 .