Carbosulfan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
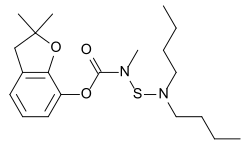
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Carbosulfan | |||||||||||||||
| other names |
2,3-dihydro-2,2-dimethyl-7-benzofuryl [(dibutylamino) thio] methyl carbamate |
|||||||||||||||
| Molecular formula | C 20 H 32 N 2 O 3 S | |||||||||||||||
| Brief description |
brown liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 380.55 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.056 g cm −3 |
|||||||||||||||
| boiling point |
124-128 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Carbosulfan is a chemical compound from the group of sulfenamides , carbamates and substituted oxygen-containing heterocycles .
Extraction and presentation
Carbosulfan can be obtained by reacting carbofuran with sulfur dichloride and dibutylamine .
properties
Carbosulfan is a brown liquid that is practically insoluble in water.
use
Carbosulfan is used as a broad spectrum insecticide similar to the related carbofuran. The effect is based on the inhibition of acetylcholinesterase . Several metabolites are produced upon breakdown, including carbofuran and dibutylamine.
Admission
In Switzerland, carbosulfan was contained in preparations against wireworms and white grubs, as well as against a number of insect pests on some vegetables and ornamentals. These products were taken off the market in May 2012, the use-by period expired in May 2013. In the EU countries including Germany and Austria as well as in Switzerland, no pesticides containing this active ingredient are currently approved.
Individual evidence
- ↑ a b c d e f g h i Entry for CAS no. 55285-14-8 in the GESTIS substance database of the IFA , accessed on May 31, 2011(JavaScript required) .
- ^ Joint Meeting on Pesticide Residues (JMPR), Monograph für Carbosulfan (1984) , accessed December 9, 2014.
- ↑ Entry on 2,3-dihydro-2,2-dimethyl-7-benzofuryl [(dibutylamino) thio] methylcarbamate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can use the expand harmonized classification and labeling .
- ↑ Thomas A. Unger, Pesticide Synthesis Handbook; P. 73; ISBN 978-0-81551401-5 .
- ^ Joint Meeting on Pesticide Residues (JMPR), Monograph for Carbosulfan (2002) , accessed December 9, 2014.
- ↑ garten.ch: Marshal / Carbosulfan no longer available , accessed on August 1, 2013.
- ↑ Directorate-General for Health and Food Safety of the European Commission: Entry on carbosulfan in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 8, 2016.

