Chlordimeform
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
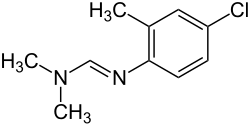
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Chlordimeform | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 13 ClN 2 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 196.68 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.10 g cm −3 |
||||||||||||||||||
| Melting point |
35 ° C |
||||||||||||||||||
| boiling point |
164 ° C (at 18.7 mbar) |
||||||||||||||||||
| Vapor pressure |
48 mPa (20 ° C) |
||||||||||||||||||
| solubility |
very heavy in water (0.25 g l −1 ) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chlordimeform is a chemical compound from the group of amidines and organic chlorine compounds .
history
Chlordimeform was sold by Ciba-Geigy between 1966 and 1976 and between 1978 and 1988 under the name Galecron as a pesticide, particularly against spider mites in cotton and other crops. In 1978, Ciba-Geigy stopped manufacturing and selling the product after independent scientists suspected the product was carcinogenic after testing on animals .
Extraction and presentation
Chlordimeform is produced industrially by condensing a Vilsmeier reagent (obtained by reacting dimethylformamide with phosphorus oxychloride , thionyl chloride or cobalt (II) chloride ) either with 4-chloro- o- toluidine or with o- toluidine and subsequent chlorination of the intermediate product formed.
use
Chlordimeform and its hydrochloride are used as pesticides ( acaricide and insecticide ). In Germany, Austria and Switzerland no pesticides with this active ingredient are approved.
Web links
- Joint Meeting on Pesticide Residues (JMPR), Monograph for Chlordimeform
Individual evidence
- ↑ a b c d e f g h Entry on Chlordimeform in the GESTIS substance database of the IFA , accessed on May 15, 2017(JavaScript required) .
- ↑ a b Environmental Health Criteria (EHC) for Cholordimeform , accessed November 19, 2014.
- ↑ Entry on Chlordimeform in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet Chlordimeform at Sigma-Aldrich , accessed on May 15, 2017 ( PDF ).
- ^ Pesticides in the North-South area of conflict, 1960s to 1980s. The example of Galecron . .
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.


