Chloridazon
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chloridazon | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 8 ClN 3 O | |||||||||||||||
| Brief description |
|
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 221.66 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.54 g cm −3 |
|||||||||||||||
| Melting point |
206 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chloridazon is a selective herbicide (pesticides) from the group of pyridazone - derivatives , which in the 1960's by BASF was brought to the market, and mainly in the beet is used. It works by inhibiting photosynthesis and the Hill reaction and is absorbed through the roots of the plants.
Since the chloridazon (in the form of the degradation product desphenyl-chloridazon ), which has been used for forty years, was detected in groundwater in 2007, the chemical industry has been recommending voluntarily since March 2007 not to use it in drinking water protection areas.
Extraction and presentation
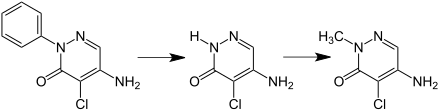
Chloridazon can be made by reacting mucochloric acid with phenylhydrazine and ammonia . Until 1996 it was contaminated to a significant extent with the ineffective isochloridazone.
Approval and use
Various plant protection products (e.g. pyramine) with this active ingredient are approved in Switzerland, but there is no approval in Germany or Austria.
Chloridazon is no longer used in the United States.
proof
Chloridazon can be detected by both gas chromatographic and liquid chromatographic methods. A mass spectrometer can also be used for detection and quantification .
Dismantling
Chloridazon becomes metabolite B1 (methyl-desphenyl-chloridazon or 1-methyl-4-amino-5-chlorpyridazin-6-one) via the metabolite B (desphenyl-chloridazon or 4-amino-5-chlorpyridazin-6-one) ) reduced.
Individual evidence
- ↑ a b c d e f Entry on Chloridazon in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Standard for chloridazon active constituent. Australian Pesticides and Veterinary Medicines Authority, August 1, 2014, accessed June 20, 2017 .
- ↑ Entry on Chloridazon. In: Römpp Online . Georg Thieme Verlag, accessed on May 16, 2014.
- ↑ record in Pesticides Database (English) .
- ↑ GWA Milne (Ed.): CRC Handbook of Pesticides . CRC Press, Boca Raton 1994, ISBN 978-0-8493-2447-5 , pp. 175 ( limited preview in Google Book search).
- ↑ Entry on Chloridazon in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet Chloridazon at Sigma-Aldrich , accessed on May 14, 2017 ( PDF ).
- ↑ a b BASF and Chloridazon. (PDF) In: Audace Association. Retrieved March 19, 2019 .
- ↑ Studies on pesticides in NRW .
- ↑ Application instructions for herbicides in beet cultivation ( Memento of September 28, 2007 in the Internet Archive ) (PDF; 190 kB).
- ↑ Industry recommends restrictions on the use of chloridazone-containing pesticides. In: bayern.de. LGL , March 15, 2007, accessed April 4, 2018 .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 519 ( limited preview in Google Book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on Chloridazon (aka pyrazone) in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 5, 2019.
- ↑ Hübschmann, Hans-Joachim .: Handbook of GC / MS: fundamentals and applications . 2nd rev. and enl. ed. Wiley-VCH, Weinheim 2009, ISBN 978-3-527-31427-0 ( limited preview in Google Book Search [accessed March 19, 2019]).
- ↑ Investigation of the herbicide Chloridazon. Retrieved March 19, 2019 .
- ↑ Plant protection product metabolites. Occurrence and evaluation (page 20) (PDF; 3.2 MB).