Conhydrin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
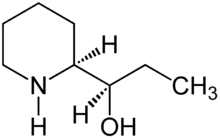
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Conhydrin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 17 NO | ||||||||||||||||||
| Brief description |
white crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 143.23 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
121 ° C |
||||||||||||||||||
| boiling point |
224-226 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
0.4 mg · kg -1 ( LD Lo , guinea pigs , sc ) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Conhydrin is a pseudo-alkaloid that was discovered by Theodor Wertheim in 1856 . It is a secondary amine , an α-substituted derivative of piperidine and contains an alcoholic hydroxyl group . In nature it occurs as (+) - conhydrin in the spotted hemlock ( Conium maculatum ) and is therefore also referred to in the literature as one of the hemlock or conium alkaloids .
Occurrence and biosynthesis
Conhydrin and its isomer pseudoconhydrin occur almost exclusively in the spotted hemlock and predominantly in its flowers. Conhydrin, pseudoconhydrin and the similar coniin and methylconiin are synthesized in the plant by first tetramerizing four C 2 units to a 3,5,7-trioxo- octanoic acid , reducing them and, after transamination, cyclizing them to γ-conicein. The plant can produce all conium alkaloids from this raw material. A previously discussed biosynthetic pathway via lysine has now been refuted.
toxicology
The toxicity of conhydrin and pseudoconhydrin is comparable. Symptoms include spastic cramps and a drop in body temperature. The lowest known lethal dose is 400 µg per kg body weight in animal experiments. Conhydrin and pseudoconhydrin are less toxic to humans than coniin. Conhydrin can arise as a by-product in the technical production of coniin.
literature
- Paul Karrer: Textbook of Organic Chemistry. 10th edition. Georg Thieme Verlag, Stuttgart 1948, p. 890.
- Entry to conium alkaloids. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
Individual evidence
- ^ Webster's Revised Unabridged Dictionary, 1913.
- ^ The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006, ISBN 0-911910-00-X , p. 420.
- ↑ Ernst Albert Schmidt: Detailed textbook of pharmaceutical chemistry. F. Vieweg & Sohn, 1896, p. 1270 ( limited preview in the Google book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on conhydrin in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ^ A b D. Bovet, F. Bovet-Nitti: Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif. S. Karger, New York 1948, p. 593.
- ↑ HW Felter, JU Lloyd: King's American Dispensatory. 18th edition. 3rd rev. Portland 1983.
- ↑ E. Glotter, L. Zechmeister: Advances in the chemistry of organic natural substances. Springer Verlag, 1971, ISBN 3-211-81024-2 .
