Cyclohexane-1,2-dicarboxylic anhydride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula of hexahydrophthalic anhydride | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cyclohexane-1,2-dicarboxylic anhydride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 10 O 3 | ||||||||||||||||||
| Brief description |
vitreous, colorless and odorless mass |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 154.17 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.19 g cm −3 |
||||||||||||||||||
| Melting point |
32 ° C |
||||||||||||||||||
| boiling point |
291 ° C |
||||||||||||||||||
| solubility |
heavy in water (4.2 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
Of particular concern: Serious effects on human health are considered likely |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
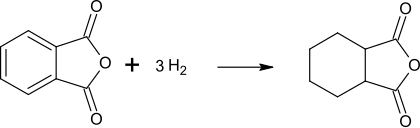
Cyclohexane-1,2-dicarboxylic acid anhydride , also hexahydrophthalic anhydride , is a chemical compound from the group of cyclic carboxylic acid anhydrides . On a ring made of cyclohexane there are two carboxylic acid groups in the ortho position, which together form an anhydride .
Extraction and presentation
Cyclohexane-1,2-dicarboxylic acid anhydride is produced via the nuclear hydrogenation of phthalic anhydride . This additional process step is one reason for the higher price compared to aromatic phthalic anhydride.
use
Cyclohexane-1,2-dicarboxylic acid anhydride is used as a monomer in various areas of polymer chemistry . So is z. B. to mention the application as an alternative to phthalic anhydride . What is desired here is better weather resistance, especially against UV light , with high hardness at the same time, which can be achieved by using hexahydrophthalic anhydride. Furthermore, with the aid of cyclohexane-1,2-dicarboxylic acid anhydride, binders, polyester resins , for paint applications with a significantly lower viscosity than those based on isophthalic acid can be produced. This results in a higher processing solids content, which is of great interest in times when much attention is paid to environmental protection . Processing solids are understood to be the non-volatile content of a paint system. Since the volatile components are mostly organic solvents ( VOC ), their share should be kept as low as possible. In addition to reducing the absolute amount of paint required or switching to aqueous systems, increasing the processing solids is the best option here.
In addition to being used as a binder in paints, cyclohexane-1,2-dicarboxylic acid anhydride can also be used as an anhydride hardener for epoxy resins . One application would be cast resin compounds which can cure at room temperature or at elevated temperatures. The higher price compared to phthalic anhydride should also be noted here.
Individual evidence
- ↑ a b c d e f g h Entry on cyclohexane-1,2-dicarboxylic acid anhydride in the GESTIS substance database of the IFA , accessed on June 28, 2018(JavaScript required) .
- ↑ Entry on cyclohexane-1,2-dicarboxylic acid anhydride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on June 29, 2018. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on April 21, 2020.
- ↑ Ulrich Poth: Synthetic binders for coating systems . Vincentz Network, Hannover 2016, ISBN 978-3-86630-611-0 , p. 60 .
- ↑ a b Ulrich Poth: Polyester and Alkyd Resins: Fundamentals and Applications. Vincentz Network, Hannover 2014, ISBN 978-3-86630-663-9 , p. 119, 122, 132 .
- ↑ Hans Domininghaus: Plastics: properties and applications . 8th, arr. Springer Berlin, Berlin 2011, ISBN 978-3-642-16172-8 , pp. 1154 .