1,2-dibromo-3-chloropropane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Basic structural formula (stereocenter is marked with an * ) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,2-dibromo-3-chloropropane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 5 Br 2 Cl | |||||||||||||||
| Brief description |
yellow-brown to brown liquid with a faint pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 236.33 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
2.08 g cm −3 (at 20 ° C) |
|||||||||||||||
| Melting point |
5 ° C |
|||||||||||||||
| boiling point |
196 ° C (110 h Pa ) |
|||||||||||||||
| Vapor pressure |
0.77 h Pa (20 ° C) |
|||||||||||||||
| solubility |
poor in water (1 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.5542 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,2-Dibromo-3-chloropropane is an active ingredient that belongs to the group of halogenated hydrocarbons and was used in nematicides, for example under the trade names Nemagon or Fumazone .
1,2-Dibromo-3-chloropropane is suspected to be responsible for the poisoning of up to 20,000 workers on banana plantations in Latin America . Male farm workers became sterile from DBCP and won the process. They were awarded $ 3.2 million in damages.
Stereoisomerism
It is an optically inactive racemate (1: 1 mixture) of the ( R ) - and ( S ) form, which can be named as follows:
- ( R ) -1,2-dibromo-3-chloropropane
- ( S ) -1,2-dibromo-3-chloropropane
| 1,2-dibromo-3-chloropropane (2 stereoisomers) |
|
|---|---|
 ( R ) configuration |
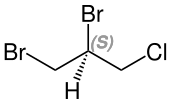 ( S ) configuration |
use
General use
From the mid-1950s to 1977, 1,2-dibromo-3-chloropropane was used in crop protection products for more than forty different plants in the United States. Preparations containing DBCP have been marketed by a number of companies, including Dow Chemical under the name Fumazone , Occidental Petroleum under the name Oxy bbc 12-e and Shell under the name Nemagon . From 1977 to 1979 the Environmental Protection Agency suspended the approval of almost all DBCP-containing preparations; it was only allowed to continue to be used for pineapple cultivation in Hawaii. In 1985 the approval for all DBCP-containing products was finally revoked and the further use of DBCP supplies was prohibited. In the European Union and Switzerland, 1,2-dibromo-3-chloropropane is also not approved as a plant protection agent.
Use in Latin America
After the Dow Chemical workers' claims for damages, who were exposed to the pesticide in production and thus became sterile, were decided in favor of the workers, the Dole Food Company recommended that DBCP no longer be used on its banana plantations in Latin America. Here, too, people were sterilized by the pesticide sprayed by planes over the banana plantations. Although they successfully sued the companies for damages, they did not get their money. A follow-up lawsuit in Los Angeles on November 5, 2007 awarded workers $ 3.2 million; the Dow and Dole companies want to challenge the judgment.
On October 9, 2019, a garnishment and transfer order was issued for parts of the Dow Olefinverbund . This is because a court in France has accepted the lawsuit of 1245 banana farmers. The trial is expected to take place in January 2020.
safety instructions
1,2-Dibromo-3-chloropropane is classified as toxic. 1,2-Dibromo-3-chloropropane can damage the genetic material, possibly cause cancer and impair fertility. Any exposure is to be avoided.
- Registry of Toxic Effects of Chemical Substances (RTECS) Number: TX8750000
acute toxicity
The acute toxicity is moderate; for inhalation a were in animal experiments with rats LC50 of 996 mg / m 3 determined; A NOAEL of 0.94 mg / m 3 was determined for the same route of administration in rabbits . For oral administration, NOAELs of 50–370 mg / kg body weight are given for various animal species; on the other hand, the NOAEL for skin absorption in rabbits is 1420 mg / kg body weight.
Reproductive toxicology
The high reproductive toxicity is much more critical. Long-term exposure to DBCP leads to fertility problems in men. This was first discovered in 1977 in chemical workers at Occidental Petroleum in the United States, many of whom became sterile from handling DBCP. Animal experiments confirm damage to the testes by DBCP. A NOAEL of 1 mg / kg body weight is given for reproductive toxicity in rats. Which way the spermatogenesis is disturbed, is not clear; an interaction of DBCP with the endocrine system similar to endocrine disruptors is discussed.
Carcinogenicity
It is unclear whether DBCP can cause cancer in humans. According to the International Agency for Research on Cancer , the data collected from several epidemiological studies are inadequate. However, there is sufficient evidence from animal studies to show that DBCP can cause cancer in several animal species. From this it is deduced that DBCP should be considered a potentially carcinogenic substance in humans. Other agencies (EPA) classify DBCP as a likely carcinogen for humans. In the more recent specialist literature it is stated that although the data in humans are still unclear, the data from animal experiments are sufficient for a justified suspicion that cancer can also be triggered in humans.
Individual evidence
- ↑ a b c d e f g h i j Entry on 1,2-dibromo-3-chloropropane in the GESTIS substance database of the IFA , accessed on February 13, 2017(JavaScript required) .
- ↑ Data sheet 1,2-dibromo-3-chloropropane from Sigma-Aldrich , accessed on March 5, 2011 ( PDF ).
- ↑ Entry on 1,2-dibromo-3-chloropropane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A b John Spano: Dole must pay farmworkers $ 3.2 million. In: Los Angeles Times. November 6, 2007 (English).
- ↑ a b EPA to DBCP (English) .
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national directory of plant protection products in Switzerland ; Retrieved April 18, 2016.
- ↑ Steffen Höhne: Dow seizure: chemical works confiscated due to lawsuit from banana farmers. In: fr.de . October 24, 2019, accessed October 28, 2019 .
- ↑ Christa Dettwiler: Plantation workers from Nicaragua are suing pesticide manufacturers. In: infosperber.ch . October 20, 2019. Retrieved October 28, 2019 .
- ↑ environmentalchemistry.com entry .
- ↑ D. Whorton, RM Krauss, S. Marshall, TH Milby: Infertility in male pesticide workers. In: Lancet. 2 (8051), Dec 17, 1977, pp. 1259-1261. PMID 73955 .
- ↑ IARC assessment of DBCP (pdf, English; 390 kB) IARC volume 71, 1999.
- ^ HA Clark, SM Snedeker: Critical evaluation of the cancer risk of dibromochloropropane (DBCP) . In: J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 23 (2), 2005, pp. 215-260. PMID 16291528 .
Web links
- Pesticide scandal: millions in compensation for impotent banana workers. In: Der Spiegel. December 21, 2007.
- environmentalchemistry.com entry
- Plantation Workers Look for Justice in the North. In: Los Angeles Times. May 27, 2007 (article about Nemagon operation in Nicaragua; English)

