Perphenazine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
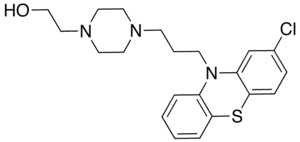
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Perphenazine | |||||||||||||||||||||
| other names |
2- {4- [3- (2-chloro-10 H -phenothiazin-10-yl) propyl] piperazin-1-yl} ethanol |
|||||||||||||||||||||
| Molecular formula | C 21 H 26 ClN 3 OS | |||||||||||||||||||||
| Brief description |
White, light-sensitive crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 403,97 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
94-100 ° C |
|||||||||||||||||||||
| boiling point |
278-281 ° C (133 Pa ) |
|||||||||||||||||||||
| solubility |
Water: 28.3 mg l −1 (24 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Perphenazine is a drug from the phenothiazine group that is used in the treatment of psychoses . Perphenazine was launched in 1957. However, due to the availability of newer neuroleptics , it is used less and less frequently. In the CATIE study , perphenazine was used as a comparison substance to the atypical neuroleptics .
Clinical information
Application areas (indications)
Areas of application are acute psychoses , for example schizophrenia , with delusions , hallucinations , catatonic syndromes , delirious and other organic psychoses , states of excitement .
Perphenazine may also be indicated for the treatment of vomiting when other treatments are impractical or unsuccessful.
Perphenazine can also be used off-label for the treatment of acute T-lymphoblastic leukemia .
Dosis, kind and Time of the Use
The dosage is 4 to 8 mg up to three times a day. Perphenazine can be used for long-term treatment, but this increases the risk of tardive dyskinesia .
Contraindications (contraindications)
Although the drug belongs to the group of phenothiazines , the range of contraindications is more similar to that of haloperidol .
Use during pregnancy and breastfeeding
Use in the first trimester is contraindicated; in the second or third trimester, the indication should be made very strict.
Adverse effects (side effects)
Although the drug belongs to the group of phenothiazines , the side effects are more similar to those of haloperidol .
Pharmacological properties
Mechanism of action (pharmacodynamics)
At the same dosage, perphenazine is 10 to 15 times more effective than the comparative neuroleptic chlorpromazine and is therefore a so-called highly potent neuroleptic. Perphenazine also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
Absorption and distribution in the body (pharmacokinetics)
The bioavailability is 40%, the plasma half-life 8–12 hours. Perphenazine is therefore usually administered two to three times a day, with the dose being concentrated towards the evening due to its sedating and blood pressure lowering effect. Perphenazine is metabolized in the liver .
Other Information
Chemical information
Perphenazine is a phenothiazine with a piperazinylalkyl side chain on ring 2. Perphenazine therefore has a structural-chemical similarity with the first neuroleptic ever discovered , chlorpromazine .
Historical information
The active ingredient became public through the use of the preparation Decentan as part of the processing of the fate of home children. According to this, drug trials are said to have been carried out on children in the late 1950s. The institution concerned replied that the drug had been used, but as part of the treatment of "particular restlessness". Whether tests have also taken place on those affected is the subject of ongoing investigations with the help of the documents sent to the Merck Group , which are now back at the affected facility.
Trade names
Decentane (D, A), Trilafon (CH), Generic (D)
Note: In Germany there is a depot form of Decentan that allows a 14-day intramuscular injection in doses of 50 to 200 mg.
See also
Individual evidence
- ^ A b c The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; ISBN 978-0-911910-00-1 .
- ↑ a b Entry on perphenazine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Data sheet Perphenazine from Sigma-Aldrich , accessed on April 18, 2011 ( PDF ).
- ↑ ePsy.de: Psychopharmaka Zeittafel
- ↑ ePsy.de: CATIE - Schizophrenia Study .
- ↑ Alejandro Gutierrez, Li Pan, Richard WJ Groen, Frederic Baleydier, Alex Kentsis: Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia . In: The Journal of Clinical Investigation . tape 124 , no. 2 , February 2014, p. 644–655 , doi : 10.1172 / JCI65093 , PMID 24401270 , PMC 3904599 (free full text).
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P: Identification of novel functional inhibitors of acid sphingomyelinase . In: PLoS ONE . 6, No. 8, 2011, p. E23852. doi : 10.1371 / journal.pone.0023852 .
- ^ SPIEGEL ONLINE, Hamburg Germany: drug tests: home children were guinea pigs. In: SPIEGEL ONLINE. Retrieved October 25, 2016 .
- ↑ Franz Sales House: News Issue. In: www.franz-sales-haus.de. Archived from the original on October 26, 2016 ; Retrieved October 26, 2016 .