Density anomaly
For most substances takes density with decreasing temperature to even a change of state of time. A chemical substance shows a density anomaly if its density decreases below a certain temperature when the temperature drops, i.e. the substance expands when it cools down (negative thermal expansion).
Density anomalies occur with the chemical elements antimony , bismuth , gallium , germanium , molten lithium , plutonium , silicon and tellurium , likewise with alloys such as Wood's metal and compounds such as zirconium tungstate ( ) , or zinc cyanide . Water is the most important substance in which such an anomaly occurs: on the one hand, the maximum density of liquid water above 0 ° C is reached, on the other hand, ice has a lower density than liquid water.
Some highly polar liquefied gases also show density anomalies, e.g. B. hydrogen fluoride and ammonia . Even if β-tin is converted into another modification (α-tin) below 13.2 ° C , its density changes, but here it is irreversible.
application
Substances with anomalous density can find their engineering application as a compensator of thermal expansion. In doing so, substances with positive thermal expansion and substances with negative thermal expansion (i.e. with density anomalies) are brought together so that when the temperature changes, expansion and contraction are balanced out and the material does not change its volume at all or changes in a precisely defined manner with temperature . Thermal expansion close to zero guarantees unchanged performance at different temperatures.
A good example from everyday life for materials with a thermal expansion close to zero are glass ceramic hobs such. B. Ceran . These have a high resistance to temperature changes , so that the glass does not crack if it is heated on one side while the other side is at room temperature . This is because some phases contained in these ceramics have an anomaly in density. The glass ceramic is adjusted with the aid of the chemical composition in such a way that the negative thermal expansion of these phases compensates for the positive thermal expansion of other phases when the temperature changes. Then the entire hob shows hardly any thermal expansion and the Ceran does not crack if it is not heated evenly everywhere.
Especially in engineering z. B. in the manufacture of precision instruments one is always looking for materials with the most constant possible performance in different temperature ranges. Materials with a density anomaly and a cubic lattice are particularly suitable , since these have isotropic negative thermal expansion, i.e. H. that their expansion is the same in all three spatial directions. Examples include , and . While over a temperature range from 0.3 to 1050 K has negative thermal expansion, is evident in and the density anomaly, however, only in their high temperature phase beginning at 350 to 400 K.
However, it can also be useful to design materials with a precisely defined thermal expansion. In the case of dental implants , it is important that the filling does not expand significantly more or less with temperature. B. takes a hot or cold drink . It can therefore be advantageous to match the overall extent of the implant to the extent of the teeth by using materials with positive and negative thermal expansion .
Examples
water
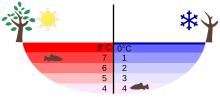
At normal pressure , water has its greatest density of approx. 1000 kilograms per cubic meter at 3.98 ° C and is liquid. Below 3.98 ° C, water expands (again) when the temperature drops (further) - even when it changes to a solid state. The anomaly of the water exists in the range between 0 ° C and 3.98 ° C, the ice does not behave abnormally, even if the density of the ice is atypically lower than that of the liquid water. The currently most accurate published values for the maximum density are (999.974950 ± 0.00084) kg / m 3 at a temperature of (3.983 ± 0.00067) ° C. The values represent an average of the figures published by various physical institutes (as of 2005).
The calculation of the density of air-free water D LF as a function of the temperature T ([ T ] = ° C) can be done with the help of the following virial equation :
- .
with the coefficients: a 0 = 999.83952; a 1 = 16.952577 (° C) -1 ; a 2 = −7.9905127 · 10 −3 (° C) −2 ; a 3 = −4.6241757 · 10 −5 (° C) −3 ; a 4 = 1.0584601 · 10 −7 (° C) −4 ; a 5 = −2.8103006 · 10 −10 (° C) −5 and b = 0.0168872. For the calculation of the density of air-saturated water is corrected to the value D LG / (g / l) = D LF / (g / l) - 0.004612 + 0.000106 (° C) -1 · T .
In the solid state of aggregation - in this case ice - a high long-range order is normally achieved through the formation of a crystal lattice in the course of crystallization . In the liquid state there is a mixture of order and chaos, with the molecules filling a larger volume due to their higher speed . So the volume increases and the density decreases. In the gaseous state the maximum disorder is reached; H. the molecules are accordingly distributed evenly over the maximum available space.
The reason for the anomaly of the water lies in the chaining of the water molecules via hydrogen bonds . As a result, the structure requires more space in the solid state than in the case of mobile molecules. Structure formation is a progressive process, which means that so-called clusters of water molecules are already present in the liquid state . At 3.98 ° C, the state is reached in which the individual clusters occupy the smallest volume and thus have the greatest density. If the temperature drops further, more volume is required due to a constant change in the crystal structures . When the temperature rises, the molecules need more freedom of movement again, which also increases the volume.
The density anomaly of the water is important for life in waters in colder climates. Surface water does not sink below a temperature of around 4 ° C. Instead of the associated cooling of deeper water layers and a complete freezing through from below, thermal layers can form. Aquatic animals and plants can survive under the ice sheet.
The temperature at which water reaches its greatest density decreases with increasing pressure from 3.98 ° C (1 bar) over approx. 2 ° C (100 bar) to approx. 0 ° C (200 bar, whereby the freezing point is itself here) has dropped to −1.5 ° C).
Numerical values for density anomaly and expansion coefficient of ice and water at normal pressure
The calculated expansion coefficient is an average expansion coefficient between the two temperatures.
| substance | / in [° C] | / in [g / cm³] | in [K] | mean temperature in [° C] | in [1 / K] | swell |
|---|---|---|---|---|---|---|
| water | 0/0 | 0.918 (ice) / 0.999840 (water) | 0 | 0 | - | , |
| 0/1 | 0.918 (ice) / 0.999899 | 1 | 0.5 | -0.0819 ! | ||
| 0/1 | 0.999840 (water) / 0.999899 | 1 | 0.5 | −0.000059006 | ||
| 1/2 | 0.999899 / 0.999940 | 1 | 1.5 | -0.0000410025 | ||
| 2/3 | 0.999940 / 0.999964 | 1 | 2.5 | -0.0000240009 | ||
| 3 / 3,983 (density maximum!) | 0.999964 / 0.999975 | 0.983 | 3.4915 | -0.0000119051 | ||
| 3/4 | 0.999964 / 0.999972 | 1 | 3.5 | -0.00000800023 | ||
| 3.983 (density maximum!) / 4 | 0.999975 / 0.999972 | 0.017 | 3.9915 | +0.000176476 | ||
| 3/5 | 0.999964 / 0.999964 | 2 | 4 (close to the maximum density!) | 0 | ||
| 4/5 | 0.999972 / 0.999964 | 1 | 4.5 | +0.00000800028 | ||
| 5/6 | 0.999964 / 0.999940 | 1 | 5.5 | +0.0000240014 | ||
| 6/7 | 0.999940 / 0.999901 | 1 | 6.5 | +0.0000390039 | ||
| 17/19 | 0.998773 / 0.998403 | 2 | 18th | +0.0001853 | ||
| 19/21 | 0.998403 / 0.997991 | 2 | 20th | +0.0002064 | ||
| 24/26 | 0.997295 / 0.996782 | 2 | 25th | +0.0002573 |
If ice melts into water at 0 ° C, its volume decreases by about 8.19%. When freezing, it increases accordingly by approx. 8.92%.
The mean expansion coefficients were calculated from the density values:
Density anomaly and (non-isobaric) expansion coefficient of liquid ammonia
At every temperature the liquid gas has a different vapor pressure , according to its vapor pressure function . Therefore, the temperature-related expansion or contraction of the volume does not take place isobarically.
The negative expansion coefficients are marked in bold.
The calculated expansion coefficient is an average expansion coefficient between the two temperatures.
| substance | / in [° C] | / in [g / cm³] | in [K] |
mean temperature in [° C] |
in [1 / K] | swell |
|---|---|---|---|---|---|---|
| liquid ammonia , boiling (at its own vapor pressure) | −70 / −68 | 0.72527 / 0.72036 | 2 | −69 | +0.003408 | |
| −68 / −66 | 0.72036 / 0.72067 | 2 | −67 | -0.000215 | ||
| −66 / −64 | 0.72067 / 0.71839 | 2 | −65 | +0.001587 | ||
| -64 / -62 | 0.71839 / 0.71608 | 2 | −63 | +0.001613 | ||
| −50 / −48 | 0.70200 / 0.69964 | 2 | −49 | +0.001687 | ||
| −30 / −28 | 0.67764 / 0.67517 | 2 | −29 | +0.001829 | ||
| −28 / −26 | 0.67517 / 0.67263 | 2 | −27 | +0.001888 | ||
| −26 / −24 | 0.67263 / 0.67463 | 2 | −25 | -0.001482 | ||
| −24 / −22 | 0.67463 / 0.68587 | 2 | −23 | -0.008194 | ||
| −22 / −20 | 0.68587 / 0.66503 | 2 | −21 | +0.015668 | ||
| −2 / 0 | 0.64127 / 0.63857 | 2 | −1 | +0.002114 | ||
| −2 / 2 | 0.64127 / 0.63585 | 4th | 0 | +0.002131 | ||
| 0/2 | 0.63857 / 0.63585 | 2 | 1 | +0.002139 | ||
| 18/20 | 0.61320 / 0.61028 | 2 | 19th | +0.002392 | ||
| 18/22 | 0.61320 / 0.60731 | 4th | 20th | +0.002425 | ||
| 20/22 | 0.61028 / 0.60731 | 2 | 21st | +0.002445 | ||
| 24/26 | 0.60438 / 0.60132 | 2 | 25th | +0.002544 | ||
| 48/50 | 0.56628 / 0.56306 | 2 | 49 | +0.002859 |
Note: Density values and expansion coefficients of liquid ammonia show two density anomalies in the temperature range under consideration!
The mean expansion coefficients were calculated from the density values:
The density quotients are indirectly proportional to the volume quotients or the quotients of the specific volumes v ( mass- specific or molar volume )!
Density anomaly and expansion coefficient of molten lithium
See: Coefficient of expansion # Numerical values of metal melts .
Individual evidence
- ↑ W. Fratzscher, HP Picht: Material data and characteristics of process engineering. Verlag für Grundstofftindustrie Leipzig, GDR 1979 / BRD 1993, data from metal melts, Lithium p. 176.
- ↑ http://iffwww.iff.kfa-juelich.de/~jones/PhysRevB.81.094202.pdf Density variations in liquid tellurium: Roles of rings, chains, and cavities, p. 1.
- ↑ a b Martin T Dove, Hong Fang: Negative thermal expansion and associated anomalous physical properties: review of the lattice dynamics theoretical foundation . In: Reports on Progress in Physics . tape 79 , no. 6 , June 1, 2016, ISSN 0034-4885 , p. 066503 , doi : 10.1088 / 0034-4885 / 79/6/066503 ( iop.org [accessed March 11, 2020]).
- ↑ Koshi Takenaka: Negative thermal expansion materials: technological key for control of thermal expansion . In: Science and Technology of Advanced Materials . tape 13 , no. 1 , February 2012, ISSN 1468-6996 , p. 013001 , doi : 10.1088 / 1468-6996 / 13/1/013001 ( tandfonline.com [accessed March 11, 2020]).
- ↑ R. Mittal, SL Chaplot: Lattice dynamical calculation of negative thermal expansion in ZrV 2 O 7 and HfV 2 O 7 . In: Physical Review B . tape 78 , no. 17 , November 7, 2008, ISSN 1098-0121 , p. 174303 , doi : 10.1103 / PhysRevB.78.174303 ( aps.org [accessed March 11, 2020]).
- ↑ Tetsuo Hisashige, Teppei Yamaguchi, Toshihide Tsuji, Yasuhisa Yamamura: Phase Transition of Zr1-xHfxV2O7 Solid Solutions Having Negative Thermal Expansion . In: Journal of the Ceramic Society of Japan . tape 114 , no. 1331 , 2006, ISSN 0914-5400 , p. 607–611 , doi : 10.2109 / jcersj.114.607 ( jst.go.jp [accessed March 11, 2020]).
- ↑ PTB reports 100 / 3-90
- ↑ Engineering ToolBox: Density and specific volume of a liquid versus change in pressure and temperature (English), 2009, accessed on December 28, 2018.
- ↑ U. Hübschmann, E. Left: Tables on chemistry. Verlag Handwerk und Technik, Hamburg 1991, ISBN 3-582-01234-4 , density of mercury and water at different temperatures and air pressure, p. 36.
- ^ Formulas and tables for secondary levels I and II. Paetec GmbH, 1996, ISBN 3-89517-253-7 , p. 11
- ↑ W. Fratzscher, HP Picht: Material data and characteristics of process engineering. Verlag für Grundstoffindindustrie Leipzig, GDR 1979 / BRD 1993, pp. 144–146 - Thermodynamic data of ammonia, density values calculated from specific volumes v '.