Double tooth consecration
| Double tooth consecration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

Double tooth harp ( Harpagus bidentatus ) |
||||||||||
| Systematics | ||||||||||
|
||||||||||
| Scientific name | ||||||||||
| Harpagus bidentatus | ||||||||||
| ( Latham , 1790) |
The double-toothed harp ( Harpagus bidentatus ), sometimes referred to as the rust-breasted goshawk , is a small bird of prey from the hawk-like family . The kindis an inhabitant of dense, natural forests in South and Central America, where they hunt insects and reptiles. What is remarkable is the hunting behavior of some specimens that have specialized in tracking groups of monkeys and consuming prey that are startled by these monkeys. Double-tooth harriers are generally not considered to be endangered at the moment, but their habitat is steadily shrinking due to the ongoing deforestation of the region's forests. Despite the large distribution area and regular sightings on gliding flights over the canopy of leaves, the species has so far been little researched.
features
Physique and appearance
With a size between 29 and 35 cm and a wingspan of 60 to 72 cm, the Doppelzahnweih is one of the smaller representatives of its family. As with many birds of prey, there is also a clear sexual dimorphism with regard to size and weight in the double-toothed consecration . Females are on average 15 to 20% larger than their male counterparts. Their weight ranges from 196 to 207 g, while males can only reach 161 to 185 g. The body is reminiscent of a typical hawk or sparrowhawk with its relatively short but broad and rounded wings, a long tail and a small, strongly curved beak . At the cutting edge of the upper mandible of the beak there are two tooth-like projections, to which the common name of the species refers and which are probably used to cut up the prey. As with the body dimensions, the color of the plumage shows a recognizable, but less pronounced sexual dimorphism. In male specimens, the back and the upper side of the wings are colored in a cloudy gray-brown. The neck, bonnet and head are a bit lighter and tend to be slightly bluish shimmering gray. The throat, on the other hand, is pearly white, with a narrow, black stripe running vertically in its center. Chest, belly and flanks as well as the feathered thighs show a reddish-brown to grayish basic color, which is interrupted by a darker sparrow coat . In some individuals, however, this pattern is so weak that it is barely recognizable or only recognizable up close. The under-tail feathers are colored white, especially when seated, they also look fluffy and are reminiscent of down . The control feathers are similarly dark in color as the back area, but show three narrow, white (inner feathers) or gray (outer feathers) bands on the upper side. On the other hand, there are only two white bands on the underside, each of which is surrounded by a broader grayish color. The underside of the wings is significantly lighter than the upper side, the hand and arm wings, like the arm covers, are colored white and lined with dark, while the hand covers are unpatterned white. In flight, this coloring creates the impression of several incomplete, dark arches on the otherwise white wings. Wax skin , reins and dark circles are yellowish and occasionally show slightly greenish tints. The iris of the eye is orange to red-orange. The beak is yellowish at the base and grayish-black in the further course. The strong, featherless legs and feet are again yellowish and end in long, strongly curved claws. Female double tooth harriers differ from male specimens mainly in the coloring on the underside. With them, the red-brown basic color is interrupted in these places by a much more pronounced, but not dark, but white sparrow coat. In addition, the back area of female birds generally looks a little lighter due to the light border of the contour feathers .
Fledglings
Doppelzahnweihe, who still wear the youth dress , are colored more brown than gray-brown on the upper side. In the neck and on the hood there is a vertical pattern of white stripes, which can be different from bird to bird. The contour feathers on the coat have paler hems. The greatest differences to the adults can be found on the underside, where the chest, the belly and, in many specimens, the flanks are still creamy white. Reddish-brown shades are only found in the area of the flanks, if at all, but are rather rare. The entire underside is also traversed by a brown stripe pattern of variable characteristics. The banding on the wings is often still incomplete and less clearly visible in flight than in older individuals.
Confusion candidates
Due to the hawk-like or sparrow-hawk-like body structure, the double-toothed harrier can easily be confused with similarly large species such as the two-colored ( Accipiter bicolor ) or the corner- tailed hawk ( A. striatus ), both of which have a very variable and confusing plumage color. However, both species have shorter wings and longer legs than the double-toothed harrier and also show much more shy and jumpy behavior. In addition, some other species come into question as possible candidates for confusion, but they can all be distinguished on the basis of more or less obvious deviations in the color of the plumage. These include, for example, the Barred Forest Falcon ( Micrastur ruficollis ), the Langschnabelweih ( Chondrohierax uncinatus ) and the Roadside Hawk ( Rupornis magnirostris ). The close resemblance to some of the larger, partially bird- eating Accipiter species could also be a form of mimicry by the double tooth harrier, which may try to deter potential predators.
Habitat and behavior
The preferred habitat of the double-toothed harrier are primary , tropical rainforests with a dense canopy of leaves and rather sparse undergrowth. Hunting attempts often take place deep inside the forest; outside of the breeding season, the species evidently avoids transitions to open terrain or secondary forest . However, more open landscapes such as bushy woodlands or mangroves are populated at least in exceptional cases or seasonally limited, as for example observations from the island of Ambergris Caye off the coast of Belize show. As with many forest-dwelling birds of prey of the Neotropics , little research has been carried out on the behavior and way of life of the double-toothed harrier . Detailed information mostly comes from a single long-term study from Guatemala, on the northern edge of the distribution area. It is therefore at least uncertain whether all known data can be transferred to the population as a whole. In resting places or during the hunt in the forest, the birds are rather inconspicuous and accordingly difficult to observe. The situation is different with the extended, morning glider flights over the canopy of leaves, which are supposed to serve to delimit one's own territory from other species. In addition, they could have a function during courtship or couple bonding. Here, the birds soar to heights of up to 300 m, only to suddenly transition into spectacular dives, in which they sometimes dive through the treetops and land within the forest. The size of the territories is only documented for a single case in which a female moved over several months in an action radius of about 2 km² around her own nesting site. Territorial conflicts between individual double teeth or pairs have not yet been directly observed. The birds are mostly sighted alone or in pairs, apparently only rarely can small, loose groups be formed. A large part of the species is very likely to be resident birds , whereby individual observations could indicate a migratory or nomadic migratory behavior of at least some individuals. So far, the aforementioned sightings of Ambergris Caye have only been made in spring. There are also well-documented individual sightings from the US states of Texas and Florida. In addition, the population that spends the first half of the year on the island of Trinidad is known to be found on the South American mainland from July to December.
Diet and hunting behavior
Double-tooth harriers are pure carnivores whose diet consists almost entirely of insects and small lizards. The exact composition of the diet can vary; Typically, however, insects make up around 60% of the diet, while lizards make up a little less than 40%. In addition, other reptiles, birds, bats and rodents are beaten when the opportunity arises. Various cicadas make up more than 80% of the insects; Grasshoppers, wasps, caterpillars, cockroaches and beetles are preyed on in significantly smaller quantities. Among the lizards, various species of the genus Anolis are particularly well represented. Older information, according to which the species almost exclusively ate birds, has been refuted as incorrect by more recent research. This assumption was probably based on a report by two bird collectors from the 1920s who had fed a captured specimen exclusively with bird carcasses for several months. All hunting attempts observed so far took place within the forest; on the other hand, the species does not seem to hunt from its own gliding flight. The preferred method of most double-toothed harriers appears to be stalking, in which the birds patiently wait for prey to pass by at a seat guard. If a suitable prey has been spotted, it will be hit with the claws after a short chase flight if it is a flying animal. If the target is on the ground or within the vegetation, the birds pounce on it with a direct, fast swing, sometimes at an acute angle. Lizards are occasionally pursued with outspread wings, running over branches and tree trunks. A rather unusual hunting method was observed in a double tooth consecration in Guatemala, which was sighted at a sinkhole while hunting bats of the genus Artibeus . This specimen started its hunt from a seat guard about 10 m below the flying bats, climbed steeply to them and finally grabbed the prey at the height of a backward roll in the air. In addition, double-tooth harriers are known to follow groups of different species of monkeys over long periods of time on their forays through the forest and to prey on insects and reptiles that have been scared off by them. For this they prefer smaller, active species such as white-shouldered capuchin ( Cebus capucinus ) and common squirrel monkey ( Saimiri sciureus ), which travel long distances in their own foraging and rarely stay longer in one and the same place. This behavior appears to be a purely parasitic relationship from which only the double consecration benefit. If they are noticed by the monkeys, they try to drive the birds away by shouting loudly and threatening gestures. If the monkeys are not active enough and scare away too little potential prey, it can happen that a double-toothed consecration hovers in the air directly above one of the monkeys and kicks it with its claws until the group starts moving again. The percentage of successful hunts found in the experiments observed so far is quite high at around 73%, but is based on comparatively little data and may therefore have to be corrected downwards in the future.
Reproduction
The incubation period varies from region to region and seems to begin towards the end of the dry season, so that the hatching of the eggs roughly coincides with the beginning of the rainy season. In Central America, double teeth consecration were observed during nest building in April and May, while in Colombia and Panama the breeding business seems to begin as early as January. The courtship behavior of the species apparently consists largely of common gliding flights at a short distance from each other, interrupted by the sudden dives described above. Before copulation, food is often passed on from the male to his partner, who sometimes also consumes it during sexual intercourse. These ritualized handovers of food are often observed during the nest building phase. After a successful hunt, the male lands about 10 to 20 m away from the nesting site and then calls the female over with a certain utterance. As a nesting place, the birds often choose trees with particularly little vegetation by tendrils and other epiphytes , which are preferably on the edges of clearings and therefore have little contact with the surrounding vegetation. The reason for this is suspected to be a reduced predation by nest robbers, as this makes it more difficult for them to climb the nesting site. However, there are apparently no special preferences for certain tree species. The nest itself is built at a height of more than 20 m in a fork of branches below the crown and consists of small sticks 20 to 40 cm in length that are arranged in a flat, cup-shaped construction. Its diameter is on average 32 cm, the depth is about 10 cm. The actual construction of the nest is almost entirely the responsibility of the female; males are rarely seen while they are contributing individual hives to the construction. After completing the nest, the female begins to lay eggs. The clutch usually consists of two eggs, more rarely a single or, in exceptional cases, three eggs. The dimensions of the eggs are only documented for a single clutch in a museum collection. They are between 36.5 and 42.1 × 31.0 and 31.6 mm. The estimated live weight is approximately 21.5 g. The base color of the shell is whitish and speckled with brown spots and spots. The incubation of the eggs is done exclusively by the female, while the male is responsible for the supply of food during this time, but never approaches the nest to more than 10 to 20 m. The female attacks possible nest predators loudly and aggressively with pursuit flights and dives until they withdraw from the vicinity of the nest. Among the possible predators of eggs and young birds are among Fischertukane ( Ramphastos sulfuratus ), Sperber consecration ( Geranospiza caerulescens ) and spider monkeys ( Ateles ). The incubation period of the clutch is about 42 to 44 days. Immediately after hatching, the young double-tooth harriers are covered in soft white down and are initially still helpless and inactive. Only after about a week do they begin to become more active overall and beg their mother for food. Especially during the cooler hours of the day and when it rains the nestlings are from female adult, brooded , in hot weather, it shields the boys on the other hand with outstretched wings from direct sunlight from. As the nestling phase progresses, the female leaves the nest for longer and longer periods of time and gradually begins to make independent attempts at hunting again. It takes an average of 27 to 37 days for the young birds to finally fled, although they can often be observed a few days in advance directly outside the nest during their first flight exercises. After leaving the nest, the offspring remain in the immediate vicinity of the nesting site for more than another month and continue to be fed there by their parents. Only then do they begin to slowly move away from the environment until they finally become completely independent and leave the territory of the adult birds permanently after a few weeks.
Utterances
Although various descriptions of calls and chants of the species exist, this is considered to be rather quiet and not very happy to call outside of the breeding season. Most often heard is a thin, high-frequency cheee - weet , which is repeated many times in succession and is intended to remind of the call of a tyrant . This utterance seems to serve primarily as a contact call between couples, but also as a begging call from brooding females before the food is handed over. A louder, sharper version of this call is also used to defend the nesting site. Males announce their arrival at the nest with a series of chirping sounds, while females in the same situation emit a short cheeep . Furthermore, a lisping tsiip - tsiip and a fast tsup - sup - sup - sup have been documented by various researchers , the exact function of which is still unclear. Nestlings beg for food with a higher, less clear variant of the typical contact call of adult birds from the adults.
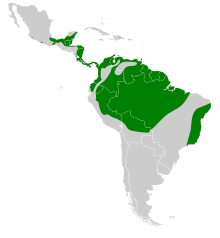
Spread and endangerment
The Doppelzahnweih is a purely neotropical species with a very large distribution area, the core of which is in the flatlands of the Amazon basin . The species is seldom sighted at altitudes above 1200 m and is locally limited, but has already been detected in Ecuador at an altitude of around 2100 m. The South American part of the range includes the northwest of Brazil, the north of Bolivia, the east of Peru and the Guyanas . In addition, all lower regions of Venezuela, Colombia and Ecuador are settled. Evidence is also known from the remains of the tropical rainforest that still exist on the Brazilian Atlantic coast. The distribution area continues over the Isthmus of Panama in a more or less wide strip along the Caribbean coast of Central America towards the north, until it finally reaches the south of Mexico. Due to its dependence on old, untouched forests for reproduction, the increasing deforestation of the region is likely to inevitably lead to a decline in the population of the double-toothed harrier. At the moment, the IUCN classifies the species at the lowest endangerment level of least concern (“not endangered”), which is mainly due to the still very large distribution area. A study from French Guiana showed an estimated population density of 15 specimens per 100 km² suitable for colonization. The IUCN's lowest estimate of the global population is 500,000 adult individuals, but the general population trend is declining. The species is listed in Appendix II of the Washington Convention on the Protection of Species .
Systematics
External system
The first description of the double tooth consecration dates back to 1790 and goes back to the British ornithologist John Latham , who described the new species using a type specimen from Cayenne in the former colony of French Guiana. As a scientific name, Latham initially gave the name Falco bidentatus , with which he placed the species, as was common with many newly described birds of prey at the time, in the genus of falcons . The specific epithet bidentatus is made up of the Latin prefix bi- for “two” and the term dentatus for “toothed”. In 1824 Nicholas Aylward Vigors described the new genus Harpagus with the double tooth consecration as a type species. The reason for this he named the morphological characteristics of the species, which are more reminiscent of a hawk or sparrowhawk than a hawk. Vigors justified the classification into a new genus mainly with the presence of the two protrusions on the beak, which do not occur in Accipiter species. Together with these, Harpagus is still in the family of the hawk-like (Accipitridae), but there belongs to the subfamily of the kites (Milvinae). The most important common feature of this widespread group are considered to be fused phalanges on the second and third toes of their feet. However, whether the Milvinae actually form a monophyletic group has not been conclusively clarified. The only other species of the genus and thus the closest relative of the Doppelzahnweih is the Braunschenkelweih ( H. diodon ). Phylogenetic analyzes indicate a separation of these two species only a short time ago at the beginning of the Pleistocene .
Internal system

Two subspecies are currently considered valid within the species . In addition to the South American nominate form H. b. bidentatus , the subspecies H. b , which is mainly widespread in Central America, still exists . fasciatus , which was originally described by the American amateur ornithologist George Newbold Lawrence as a separate species within the genus Harpagus . The boundary of the distribution areas of the two forms runs through Ecuador and Colombia.
- H. b. bidentatus ( Latham , 1790) - The nominate form occurs in the entire South American part of the distribution area as far as eastern Colombia and eastern Ecuador.
- H. b. fasciatus Lawrence , 1869 - Distributed from the western regions of Colombia and Ecuador via Central America to southern Mexico, roughly at the level of the states of Jalisco and southern Veracruz . Representatives of this subspecies show a much more pronounced sparrowing in the chest and abdominal area. In addition, the plumage of the plumage is reddish in many specimens only on the flanks and shoulders. The chest and especially the belly, on the other hand, are more whitish to cream-colored.
literature
- Mark D. Schulze, José L. Cordóva, Nathaniel E. Seavy, David F. Whitacre: Neotropical Birds of Prey: Biology and Ecology of a Forest Raptor Community . Ed .: David F. Whitacre. Cornell University Press, Ithaka / London 2012, ISBN 978-0-8014-4079-3 , pp. 68-81 .
Web links
- Recordings of calls and chants at xeno-canto.org
Individual evidence
- ↑ a b Rust-breasted goshawk ( Harpagus bidentatus ) at Avibase; accessed on 2021-07-28.
- ↑ James Ferguson-Lees, David A. Christie: Raptors of the World . Christopher Helm, London 2001, ISBN 0-7136-8026-1 , pp. 106 .
- ↑ a b c d e f g h i j k James Ferguson-Lees, David A. Christie: Raptors of the World . Christopher Helm, London 2001, ISBN 0-7136-8026-1 , pp. 366-367 .
- ↑ a b c d e f g Richard O. Bierregaard, Jeffrey S. Marks, Guy M. Kirwan: Double-toothed Kite (Harpagus bidentatus). In: Birds of the World. 2020, accessed on July 27, 2021 .
- ↑ Schulze et al., Neotropical Birds of Prey , p. 73
- ↑ Steve NG Howell, Barbara A. Dowell, Douglas A. James, Robert A. Behrstock, Chandler S. Robbins: New and noteworthy bird records from Belize . In: Bulletin of the British Ornithologists' Club . tape 112 , no. 4 , 1992, pp. 235-244 .
- ↑ Julio AB Monsalvo: Geographical variation and current knowledge on breeding patterns of Neotropical accipitrid raptors . Ed .: Universidade de Brasília. 2018.
- ↑ Schulze et al., Neotropical Birds of Prey , p. 78
- ↑ Steve NG Howell, Ian Lewington, Will Russell: Rare Birds of North America . Princeton University Press, Princeton / Oxford 2014, ISBN 978-0-691-11796-6 , pp. 230 .
- ^ Bev Hansen, Andrew W. Kratter: First Florida record of Double-toothed Kite (Harpagus bidentatus) . In: Florida Field Naturalist . tape 47 , no. 3 , 2019, p. 99-104 .
- ↑ Schulze et al., Neotropical Birds of Prey , pp. 69-72
- ↑ Aaron J. Baker, David F. Whitacre, Oscar A. Aguirre-Barrera, Juventino López Ávila, Clayton M. White: Observation of a Double-toothed Kite (Harpagus bidentatus) hawking bats . In: Journal of Raptor Research . tape 33 , no. 4 , 1999, p. 343-344 .
- ^ Roy Fontaine: Observations on the Foraging Association of Double-Toothed Kites and White-Faced Capuchin Monkeys . In: The Auk . tape 97 , no. 1 , 1980, p. 94-98 , doi : 10.1093 / auk / 97.1.94 .
- ↑ Schulze et al., Neotropical Birds of Prey , p. 72
- ↑ a b Schulze et al., Neotropical Birds of Prey , p. 75
- ↑ Schulze et al., Neotropical Birds of Prey , pp. 73-75
- ^ Robert M. Laughlin: A Nesting of the Double-Toothed Kite in Panama . In: The Condor . tape 54 , no. 3 , 1952, pp. 137-139 , doi : 10.2307 / 1365063 .
- ↑ Schulze et al., Neotropical Birds of Prey , pp. 75-78
- ↑ a b Harpagus bidentatus in the Red List of Threatened Species of the IUCN 2020.2. Listed by: BirdLife International, 2020. Retrieved July 28, 2021.
- ↑ Heather RL Lerner, David P. Mindell: Phylogeny of eagles, Old World vultures, and other Accipitridae based on nuclear and mitochondrial DNA . In: Molecular Phylogenetics and Evolution . tape 37 , no. 2 , 2005, p. 327-346 , doi : 10.1016 / j.ympev.2005.04.010 .
- ↑ David P. Mindell, Jérôme Fuchs, Jeff A. Johnson: Phylogeny, Taxonomy, and Geographic Diversity of Diurnal Raptors: Falconiformes, Accipitriformes, and Cathartiformes . In: J. Sarasola, J. Grande, J. Negro (Eds.): Birds of Prey . Springer, 2018, ISBN 978-3-319-73744-7 , pp. 3-32 , doi : 10.1007 / 978-3-319-73745-4_1 .