Ergometrist
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
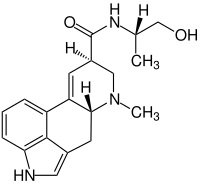
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Ergometrist | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 19 H 23 N 3 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 325.41 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
162 ° C |
|||||||||||||||||||||
| pK s value |
6.8 (at 20 ° C) |
|||||||||||||||||||||
| solubility |
Easily soluble in short-chain alcohols , ethyl acetate , acetone ; more soluble in water than the other main ergot alkaloids; sparingly soluble in chloroform |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ergometrin , also called Ergonovin or Ergobasin , is a naturally occurring chemical compound from the group of ergot alkaloids .
Occurrence
Ergometrine is one of the main alkaloids that is produced by the ergot fungus Claviceps purpurea and other members of the Clavicipitaceae family and can also be detected in various bindweed plants .
Its isolation from ergot was first described by Arthur Stoll in 1935 .
use
This alkaloid is used in obstetrics because of its uterine contracting effect. Due to the risk of permanent contractions, its use is limited to the postpartum period to loosen the placenta , to stop bleeding after loosening the placenta and to regret the uterus in the puerperium . Although ergometrine is included in the WHO list of indispensable drugs , this substance, unlike its derivative methylergometrine , no longer plays a therapeutic role in the western world.
Individual evidence
- ^ A b The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; ISBN 978-0-911910-00-1
- ↑ a b Entry on ergometrine in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ a b Ergonovine maleate salt data sheet from Sigma-Aldrich , accessed on March 30, 2011 ( PDF ).
- ^ Stoll A: The new ergot alkaloid . In: Science . 82, August, pp. 415-417.
- ^ WHO Model Lists of Essential Medicines. 15th edition. March 2007 (PDF, English; 379 kB)

