Ethyl lactate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
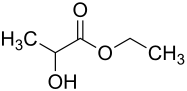
|
|||||||||||||||||||
| Structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ethyl lactate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 10 O 3 | ||||||||||||||||||
| Brief description |
colorless, mild smelling liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 118.13 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.03 g cm −3 |
||||||||||||||||||
| Melting point |
−25 ° C |
||||||||||||||||||
| boiling point |
154 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
hydrolyzed in water |
||||||||||||||||||
| Refractive index |
1.4130 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Ethyl lactate , also known as ethyl lactate and 2-Hydroxypropionsäureethylester referred to, the ethyl ester of lactic acid .
Lactic acid ethyl ester is contained in small quantities as a flavoring or fragrance, for example in wine and various fruits . Its smell is pleasantly mild and is described as fruity reminiscent of coconut .
properties
Stereoisomers
The commercially available, biotechnologically produced ethyl lactate is in the L form and bears the exact name ( S ) -2-hydroxypropionic acid ethyl ester or L - (-) - lactic acid ethyl ester. In contrast, technically produced ethyl lactate is a racemate before a 1: 1 mixture of D - (+) - ethyl lactate and L - (-) - ethyl lactate. This mixture is also called DL - (±) -lactic acid ethyl ester.
| Enantiomers of ethyl lactate | ||||
| Surname | L - (-) - ethyl lactate | D - (+) - ethyl lactate | ||
| other names | Ethyl ( S ) -2-hydroxypropionate | Ethyl ( R ) -2-hydroxypropionate | ||
| Structural formula |

|

|
||
| CAS number | 687-47-8 | 7699-00-5 | ||
| PubChem | 92831 | 637513 | ||
| ECHA info card | 100.010.632 | 100.156.709 | ||
| EC number | 211-694-1 | 628-439-9 | ||
Safety-related parameters
Ethyl lactate is considered a flammable liquid. Flammable vapor-air mixtures can form above the flash point . The compound has a flash point of 46 ° C. The explosion range is between 1.5 vol.% (70 g / m 3 ) as the lower explosion limit (LEL) and 11.4 vol.% As the upper explosion limit (UEL). The limit gap width was determined to be 0.99 mm. This results in an assignment to explosion group IIA. The ignition temperature is 400 ° C. The substance therefore falls into temperature class T2.
Manufacturing
The starting materials for the industrial production of ethyl lactate are lactic acid and ethanol . Both starting materials can be obtained from renewable raw materials such as corn . Lactic acid ethyl ester is very easily biodegradable. There is therefore growing interest in this solvent, which can be used, for example, for stripping or cleaning circuit boards in electronics .
Ecological data
- Biodegradation: 86% / 28 d, easily biodegradable
- Bioaccumulation is not to be expected
- Fish toxicity LC 50 = 320 mg / l / 96 h Brachidonia rerio
- Daphnia toxicity EC 50 = 683 mg / l / 48 h Daphnia magna
- Algae toxicity IC 50 = 2200 mg / l / 48 h Selenastrum capricornutum
- Chemical oxygen demand (COD): 1.62 g / g
use
Because of its low toxicity and favorable environmental properties, the use of ethyl lactate is increasing steadily in very different areas. For example, it is used in pharmaceutical formulations, food additives and fragrances.
Photoresists in microelectronics often contain ethyl lactate as a solvent. The name Safer Solvent for ethyl lactate has become established there.
As a solvent, it is used to dissolve nitrocellulose , cellulose acetate and cellulose ethers .
Shampoos for dogs and cats partly contain ethyl lactate in a washing active suspension . Further application possibilities will be offered in the future, for example, in the area of adhesives , food emulsifiers , as solvents for chiral syntheses, paint removers (paint strippers), etc. For some applications, the price of ethyl lactate is currently too high. However, intensive work is being carried out on more favorable manufacturing processes.
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on ethyl lactate in the GESTIS substance database of the IFA , accessed on January 15, 2020(JavaScript required) .
- ↑ Entry on lactic acid ester. In: Römpp Online . Georg Thieme Verlag, accessed on August 2, 2018.
- ↑ Entry on ethyl lactate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b c E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ ADM, leading manufacturer of ethyl lactate, accessed on July 12, 2014 ( Memento of the original from July 14, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ Data sheet ethyl lactate at Syskem.de, accessed on January 23, 2016.
- ↑ a b Ethyl Lactate Solvents: Low-Cost and Environmentally Friendly ( Memento of December 4, 2011 in the Internet Archive ). Argonne National Laboratory, accessed June 5, 2007.
- ↑ ewire.com: Cargill Dow and Ashland Sign Ethyl Lactate Agreement: Green Solvents from Renewable Resources , accessed on July 5, 2007 ( Memento of the original from September 28, 2007 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked . Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ Manufacture of semiconductor components ( Memento from September 27, 2011 in the Internet Archive ) (PDF; 575 kB).
- ↑ Fujifilm-ffem.com: HPR 500 Series , accessed July 5, 2007 .
- ↑ Lactaderm: antibacterial shampoo with ethyl lactate. ( Memento of the original from July 14, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved July 5, 2007.


