Fenofibrate
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
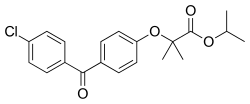
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Fenofibrate | |||||||||||||||||||||
| other names |
2- [4- (4-chlorobenzoyl) phenoxy] -2-methylpropionic acid isopropyl ester ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 20 H 21 ClO 4 | |||||||||||||||||||||
| Brief description |
white to almost white, crystalline powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 360.83 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
80–81 ° C (crystals from 2-propanol ) |
|||||||||||||||||||||
| solubility |
practically insoluble in water, sparingly soluble in methanol and ethanol , soluble in acetone , diethyl ether , benzene and chloroform |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Fenofibrate is an active ingredient from the group of fibrates (fibric acids and their derivatives) and is used in the treatment of primary and secondary hyperlipoproteinemia (as well as hypercholesterolaemia and hypertriglyceridaemia ). Fenofibrate (trade name Lipanthyl ) was patented in 1972 as a lipid lowering agent by Fournier Pharma (now Abbott Laboratories ) and is commercially available as a generic.
Pharmacological properties, side effects and contraindications
By influencing the transcription factors via the PPAR α receptor, the activity of the lipoprotein lipase in the periphery is increased, whereby the plasma concentration of triglycerides and LDL decreases, while the concentration of HDL (colloquially the "good cholesterol") increases.
Studies ( FIELD 2007, ACCORD 2008) have shown that diabetic retinopathy slows down the progression and reduced the need for laser coagulation regardless of the lipid level . In Australia, fenofibrate has been approved for this indication.
With a half-life of up to 21 hours and plasma protein binding of 99%, it is the longest acting fibrate. Possible side effects are nausea , vomiting , ventricular cardiac arrhythmias , increase in liver enzymes (ASAT / ALAT) and, rarely, myalgia and rhabdomyolysis (especially when combined with statins). Contraindications are pregnancy and breastfeeding , primary biliary cirrhosis and the use of statins.
Trade names
Fenoglide, Lipanthyl, Lipcor, Lipofen, Lofibra, Procetofen, Secalip Supra (Spain), Tricor, Triglide, Lipsin
Virological research
In July 2020, research teams from Israel and the USA reported that fenofibrate counteracts the changes in lipid metabolism caused by SARS-CoV-2 in vitro . Among other things, the accumulation of phospholipids in lung cells could be inhibited by fenofibrate and glycolysis could be increased. These effects led to an inhibition of virus replication and the virus could no longer be detected after five days with the methods used.
Web links
Individual evidence
- ↑ a b c Datasheet Fenofibrate from Sigma-Aldrich , accessed on March 31, 2011 ( PDF ).
- ^ A b c The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; ISBN 978-0-911910-00-1 .
- ^ David RP Guay: Update on fenofibrate . In: Cardiovascular Drug Reviews . 20, No. 4, 2002, pp. 281-302. doi : 10.1111 / j.1527-3466.2002.tb00098.x . PMID 12481201 .
- ^ AC Keech et al .: Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomized controlled trial , In: The Lancet , 2007, 370: 1687-1697
- ↑ Nentwich et al., Diabetes und Auge, Der Diabetologe, Springer 2014, accessed March 2, 2014
- ↑ Avner Ehrlich, Skyler Uhl, Konstantinos Ioannidis, Matan Hofree, Benjamin R.tenOever, Yaakov Nahmias: The SARS-CoV-2 Transcriptional Metabolic Signature in Lung Epithelium In Cell Metabolism , 2020