Fluorenol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
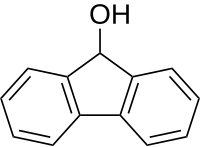
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Fluorenol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 10 O | ||||||||||||||||||
| Brief description |
whitish powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 182.22 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
153-157 ° C |
||||||||||||||||||
| boiling point |
367.5 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fluorenol is a chemical compound that belongs to the group of alcohols . It is a derivative of fluorene .
presentation
Fluorenol can be prepared by reducing fluorenone with sodium borohydride .
use
Fluorenol was patented as an insecticide in 1939 .
In animal experiments, a stimulating effect, somewhat stronger than that of the drug Modafinil , with an even lower dependency potential could be determined. Together with Modafinil, Adrafinil , Armodafinil and Fladrafinil, it is one of the eugeroika (stimulants). Further developments on the part of the US pharmaceutical company Cephalon on the possible use of the substance as an awake drug have, however, been discontinued. Fluorenol is marketed as an unregulated chemical on the Internet as a neuro-enhancer under the name Hydrafinil .
toxicity
Fluorenol has been shown to be toxic to various aquatic organisms in higher concentrations. Harmful effects on humans have not yet been investigated.
Individual evidence
- ↑ a b c d Hydrafinil (Fluorenol): A Modafinil Analogue with Questionable Safety , Mental Health Daily, accessed October 6, 2019
- ↑ a b c d e Entry for CAS no. 1689-64-1 in the GESTIS substance database of the IFA , accessed on October 6, 2019(JavaScript required) .
- ↑ a b Entry on fluorenol in the ChemSpider database of the Royal Society of Chemistry , accessed on October 6, 2019.
- ↑ C. Susana Jones: A Synthesis of 9-Fluorenol: Sodium Borohydride Reduction of 9-Fluorenone , in: Journal of Chemical Education , 1994
- ↑ a b U.S. Patent US2197249A , accessed October 6, 2019
- ↑ Clifford Fong: Modafinil and modafinil analogues: free radical mechanism of the eugeroic and cognitive enhancment effect , in: ResearchReport / Eigenenergy , 2018
- ↑ K. Hari Kumar, Mitta Srija, DK Sandeep, Ramisetty Davarika, Gunda Sai Mounica: Nootropics - Memory Boosters , in: Journal of Pharmaceutical Biology , 2016
