Fluxofenim
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
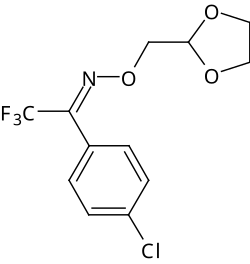
| Structural formula of the ( E ) isomer | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Fluxofenim | |||||||||||||||
| other names |
4'-chloro-2,2,2-trifluoroacetophenone- ( EZ ) - O -1,3-dioxolan-2-ylmethyloxime |
|||||||||||||||
| Molecular formula | C 12 H 11 ClF 3 NO 3 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 309.67 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.36 g cm −3 |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Fluxofenim is a chemical compound from the group of oxime ethers .
Extraction and presentation
Fluxofenim can be obtained by reacting chlorobenzene with trifluoroacetyl chloride , hydroxylamine hydrochloride and bromomethyl-1,3-dioxolane .
properties
Fluxofenim is a colorless liquid that is practically insoluble in water.
use
Fluxofenim is used as a herbicide safener on corn and sorghum . A seed dressing with 0.05–0.3% safener protects against the herbicide metolachlor . It was developed by Ciba-Geigy , tested for the first time in 1982 and published in 1986.
In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c d e Entry on Fluxofenim in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on August 1, 2013.
- ↑ a b c d e Fluxofenim data sheet at Sigma-Aldrich , accessed on May 22, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 151 ( limited preview in Google Book search).
- ↑ Patent EP0986955A1 : Glutathione conjugates as signaling molecules , Table 9.2 Safeners for sorghum and maize seed treatments .
- ↑ VSP Rao, Vallurupalli Sivaji Rao: Principles of Weed Science . Science Publ., 2000, ISBN 1-57808-069-X , pp. 114 ( limited preview in Google Book search).
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds . Wiley-VCH, 2011, ISBN 978-3-527-32965-6 , pp. 379, 389 ( limited preview in Google Book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.

