Gonosome
The sex chromosome or gonosome (sometimes also heterochromosome , heterosome or allosome ) are chromosomes whose karyotype determines the genetic sex of an individual.
Sex chromosomes do not form a homologous pair in either sex, but differ significantly in their content. Often the sex chromosomes also differ in size. The human Y chromosome is much smaller than the X chromosome , whereas the Y chromosome is significantly larger in the white carnation . Non-sex-determining chromosomes, the autosomes , are found in diploid cells as pairs of almost identical, homologous chromosomes.
If sex is determined chromosomally, it is inherited according to Mendel's rules . This form of sex determination has arisen independently of one another in the course of evolution in different groups of species and occurs, for example, in mammals, birds and some insects, but also in vascular plants . In other species, however, the sex is determined by environmental conditions, e.g. B. by the temperature during embryonic development (see sex determination ).
XY / XX system
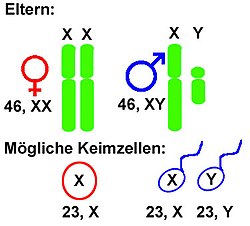
All mammals (and therefore humans) have an XY system (in the broader sense ); some lizards , amphibians and fish each ; the fruit fly Drosophila melanogaster ; as well as some plants such as the dock and some light carnations ( Silene ).
In an XX / XY system (XY system in the narrower sense), females have the same sex chromosome twice, namely two X chromosomes . They are therefore homozygous for the gonosomes . Males, on the other hand, have an X chromosome and a Y chromosome . This condition is called hemizygous . An X chromosome is always passed on from the mother, and either an X or a Y chromosome from the father. All other chromosomes, the autosomes , exist in two copies each. Most mammals have this system ( theria : marsupials and placentas , i.e. the higher mammals including humans; but not the monotremata , i.e. egg-laying mammals).
For humans , it could be shown on the basis of patients with a different number of chromosomes that whether or not a Y chromosome is present is important for gender expression. The SRY gene is located on this . It is important in the formation of a male genital tract. If there is no SRY gene, its effect can be fully or partially compensated for by SOX9 and other genes involved. In the absence of both genes, according to the results of a study, the FOXL2 gene becomes active and ensures the development of a female genital tract. In Turner syndrome , those affected have only one X chromosome and no Y chromosome. In many cases they develop a female genital tract.
In the line of development leading to humans, the sex chromosomes formed 300 to 200 million years ago in the Permian / Triassic .
In some species there are several different X chromosomes and / or several different Y chromosomes. As an extreme example, can platypus as eggs Understanding mammalian apply (scientifically monotremes). In this case, the females have ten X chromosomes (X1-X5, twice each) and the males five different X and five different Y chromosomes. The sex chromosomes of the platypus on the one hand show no homologies to those of the marsupials and placentas (higher mammals, scientifically Eutheria) on the other hand, so that it can be assumed that an XY system for sex determination within the mammals has arisen twice.
In Drosophila , individuals with an X and no Y chromosome develop into phenotypic, but sterile males. The ratio of autosomes to X chromosomes is crucial here. If there is an X chromosome for each set of autosomes, the individual is female; if there is only one X chromosome for two sets of autosomes, the individual is male. XXY individuals develop intersexes with male and female characteristics distributed like a mosaic (so-called “salt and pepper pattern”).
ZW / ZZ system
In the ZW / ZZ system, conversely, the females are hemizygous, they have a W and a Z chromosome , while the males have two Z chromosomes. It occurs in birds , most snakes , and a few lizards, fish, and amphibians each. Butterflies , caddis flies and strawberries also have a ZW system.
A likely candidate for the sex-determining gene in birds is DMRT1 , which is found on the Z chromosome but not on the W chromosome. Females have only one, but males two copies. The latter leads to a double dose of DMRT1 expression , which in turn could lead to the development of male sexual organs. DMRT1 is also increasingly expressed in the male gonads in temperature-dependent sex determination in turtles and alligators . DMRT1- homologous genes also play different roles in the sex differentiation in humans, mice, Drosophila and Caenorhabditis elegans .
XX / X0 system
In the XX / X0 system, females usually have two X chromosomes (XX), while males only have one (X0, pronounced “x-zero”). There is no other sex chromosome, so the males have one chromosome less. This type of sex determination can be found, for example, in many insects , such as fish (Zygentoma), cockroaches (Blattodea), most stoneflies (Plecoptera), dragonflies (Odonata), dust lice (Psocoptera), beaked flies (Mecoptera), grasshoppers (Orthoptera), stick insects (Phasmatodea) and Mantis (Mantodea), but also in some mayflies (Ephemeroptera) and beetles (Coleoptera). The ratio of autosomes to X chromosomes is crucial here. Two sets of autosomes and two X chromosomes result in females; two sets of autosomes and only one X chromosome result in fertile males. In contrast to Drosophila in the XY / XX system, in which a set of autosomes without a gonosome leads to the formation of sterile males.
In the XX / X0 system, males can arise despite parthenogenesis . They arise when there is an uneven distribution of the X chromosomes due to non-disjunction . This can happen in apomictic parthenogenesis during meiosis or in automictic parthenogenesis during the metaphase of mitosis due to the failure of sister chromatids to separate. This creates females with trisomal X chromosomes (XXX) and phenotypic males with one X chromosome (X0). In these cells, all cells are phenotypically male, but since they are genotypically not identical to real males due to the chromosome distribution, they are referred to as intersex males. Such males are fertile, but do not produce male offspring.
The nematode Caenorhabditis elegans has the two sexes hermaphrodite and male. While the hermaphrodites have two X chromosomes (XX), the rarely occurring males have only one of them (X0). The males in this case have 9 instead of 10 chromosomes.
Haplodiploidy
In haplodiploidy, sex is determined by the chromosomes present, but there are no sex chromosomes. In over 2000 species of hymenoptera ( ants , bees , wasps ), males hatch from unfertilized eggs, which are therefore haploid . Accordingly, they only have half as many chromosomes as diploid females (see also parthenogenesis ). In the case of the well-studied honey bees, it has been found that, similar to humans, a certain gene is ultimately decisive for determining sex. If it is available in two different versions (in the case of the fertilized eggs), females arise. If there is only one version (in the case of unfertilized eggs), males result. By inbreeding can to come that this gene is present in fertilized eggs in two identical versions. Then diploid males emerge. However, these are eaten by the workers after they hatch from the egg. Haplodiploidy has also been described in other animal groups (see main article haplodiploidy ).
Consequences of hemizygosity
While mammalian females have two X chromosomes, the males only have one X and one Y chromosome each, as just described, they are hemizygous . As a result, if there is a genetic defect on the only existing X chromosome, it cannot be caught by a functioning copy on the other chromosome, as is the case with females. Therefore, there are a number of hereditary diseases in humans that practically only occur in men. The best-known examples are a form of hemophilia , Duchenne muscular dystrophy and red-green blindness .
Conversely, in animal species with the ZW / ZZ system, the female animals are more frequently affected by sex-linked hereditary diseases, as they only have one copy of the Z chromosome.
Dose compensation

As a further consequence of the chromosomal sex determination, one chromosome is present twice in one of the sexes and only present once in the other. In order to prevent that twice as much gene product is produced here as in the opposite sex, different groups of animals have developed different strategies for "dose compensation". Dose compensation itself is not gender determining.
In humans, mice, cats and possibly mammals in general, one of the two female X chromosomes is inactivated. The inactive X chromosome undergoes a number of changes that make it a Barr body that can be detected by light microscopy (see illustration). This epigenetic process is described in detail in the articles X-Inactivation and Sex Chromatin .
In the Caenorhabditis elegans worm , on the other hand, both X chromosomes are evenly downregulated in the hermaphrodite . In the fruit fly Drosophila melanogaster there is no X-inactivation. Instead, the single X chromosome in the male is read twice as strongly as in the female.
In birds, the processes of dose compensation are not yet fully understood. Apparently, there is no compensation for some genes on the Z chromosome, so that they are more strongly expressed in males than in females. A majority of the genes with compensation are located in the MHM region of the Z chromosome (MHM from English male hypermethylated region ). In females, this region is covered by a non-coding MHM RNA and rich in a specific form of histone that promotes gene expression (H4K16ac).
Variations in the number of sex chromosomes
A number of deviations in the number of sex chromosomes are known in humans, for example in Turner syndrome or Klinefelter syndrome . Since all but one of the X chromosomes are (largely) inactivated, excess or missing X chromosomes are more tolerable than additional autosomes. Human Y chromosomes contain only very few genes, so that deviations in the number can also be tolerated here. The section Deviations in the number of sex chromosomes in the article Chromosome gives an overview of the corresponding syndromes .
Individual evidence
- ^ Brian Charlesworth: The evolution of sex chromosomes. In: Science , Vol. 251 (1991), No. 4997, pp. 1030-1033, doi: 10.1126 / science.1998119
- ↑ a b c Panagiota Manolakou, Giagkos Lavranos and Roxani Angelopoulou. Molecular patterns of sex determination in the animal kingdom: a comparative study of the biology of reproduction. 2006. Reproductive Biology and Endocrinology, 4:59. doi: 10.1186 / 1477-7827-4-59
- ↑ Navajas-Pérez et al. : The evolution of reproductive systems and sex-determining mechanisms within Rumex (Polygonaceae) inferred from nuclear and chloroplastidial sequence data. In: Molecular Biology and Evolution . Volume 22, No. 9, 2005, pp. 1929-1939, doi: 10.1093 / molbev / msi186 , PMID 15944442 .
- ↑ F. Monéger, N. Barbacar, I. Negrutiu: dioecious Silene at the X-road: the reasons Y. In: Sex Plant Reprod. Volume 12, No. 4, 2000, pp. 245-249, doi: 10.1007 / s004970050009 .
- ↑ N. Henriette Uhlenhaut, Susanne Jakob, Katrin Anlag, Tobias Eisenberger, Ryohei Sekido: Somatic Sex Reprogramming of Adult Ovaries to Testes by FOXL2 Ablation . In: Cell . tape 139 , no. 6 , December 2009, p. 1130–1142 , doi : 10.1016 / j.cell.2009.11.021 ( elsevier.com [accessed August 3, 2020]).
- ^ Voss, Heinz-Jürgen (2010): Making Sex Revisited: Deconstruction of Gender from a Biological-Medical Perspective. Transcript-Verlag, Bielefeld.
- ↑ Lizzie Buchen: The fickle Y chromosome. In: Nature , Volume 463, 2010, p. 149, doi: 10.1038 / 463149a , full text
- ↑ Frank Grützner, Willem Rens, Enkhjargal Tsend-Ayush, Nisrine El-Mogharbel, Patricia CM O'Brien, Russell C. Jones, Malcolm A. Ferguson-Smith and Jennifer A. Marshall Graves. In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. 2004. Nature 432 (7019), pp. 913-917. doi: 10.1038 / nature03021
- ↑ a b c d Payer B, Lee JT: X Chromosome Dosage Compensation: How Mammals Keep the Balance . In: Annual review of genetics . August 2008. doi : 10.1146 / annurev.genet.42.110807.091711 . PMID 18729722 .
- ↑ Traut, W., K. Sahara, F. Marec: Sex Chromosomes and Sex Determination in Lepidoptera. In: Sexual Development. Volume 1, 2008, pp. 332-346, doi: 10.1159 / 000111765 .
- ^ GM Darrow: The strawberry: history, breeding and physiology Holt, Rinehart & Winston. New York 1966
- ^ Heath Blackmon Laura Ross Doris Bachtrog: Sex Determination, Sex Chromosomes, and Karyotype Evolution in Insects . Journal of Heredity, Volume 108, Issue 1, January 1, 2017, pp. 78–93
- ↑ Thies H. Büscher : Gynandromorphism - half man, half woman - curious hybrid beings; a report with a particular focus on Gynander in the order Phasmatodea (Insecta) . Arthropoda Popularis, 3/4 2015, ZAG Wirbellose eV, Dessau-Roßlau 2015, pp. 26–37, ISSN 2190-3476
- ↑ JE. Mank and H. Ellegren: Sex linkage of sexually antagonistic genes is predicted by female, but not male effects in birds. In: evolution. 63, 2009, pp. 1464-1472, doi: 10.1111 / j.1558-5646.2009.00618.x .
- ↑ TF. Wright et al. Sex-linked inheritance of hearing and song in the Belgian Waterslanger canary. In: Proc R Soc Lond 2004, B (Suppl.) 271, S409-S412, PMC 1810118 (free full text).
- ↑ PR. Baverstock et al. A sex-linked enzyme in birds — Z-chromosome conservation but no dosage compensation . In: Nature 1982, 296: 763-6, doi: 10.1038 / 296763a0 .
- ↑ Marina Dominguez-Steglich, Michael Schmid: Sex-Linkage of the Chicken Ornithine Transcarbamylase Gene. In: Hereditas. 118, 1993, pp. 1-5, doi: 10.1111 / j.1601-5223.1993.t01-3-00001.x .
- ↑ Peter Spork-Frischling: How the excess X chromosome goes down. In: Newsletter Epigenetics. October 15, 2015, accessed April 25, 2018 .
- ↑ Hendrik Marks, Hindrik HD Kerstens, Tahsin Stefan Barakat, Erik Splinter, René AM Dirks, Guido van Mierlo, Onkar Joshi, Shuang-Yin Wang, Tomas Babak, Cornelis A. Albers, Tüzer Kalkan, Austin Smith, Alice Jouneau, Wouter de Laat, Joost Gribnau, Hendrik G. Stunnenberg: Dynamics of gene silencing during X inactivation using allele-specific RNA-seq . In: Genome Biology . tape 16 , 2015, p. 149-169 , doi : 10.1186 / s13059-015-0698-x , PMID 26235224 .
- ↑ Laura Bisoni, Laura Batlle-Morera, Adrian P. Bird, Miho Suzuki and Heather A. McQueen. Female-specific hyperacetylation of histone H4 in the chicken Z chromosome. 2005. Chromosome Res. 13 (2): 205-14. doi: 10.1007 / s10577-005-1505-4