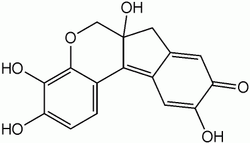
Hematein
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Hematein | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 12 O 6 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 300.26 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Hematein is a chemical compound from the group of neoflavonoids . It is the oxidation product of hematoxylin . In the presence of aluminum ions, it binds to nucleic acids . This stains DNA in cell nuclei and RNA in the cytosol . It also binds to keratohyalin granules and hydroxyapatite . An analogue of hematein is Brazilein .
properties
In the freshly precipitated state, hematein appears as a swollen precipitate of red-brown color. When dry, it is dark green, shiny metallic and translucent in red in thin layers, its coating powder is reddish brown. It dissolves slowly in cold water and more easily in boiling water. It initially dissolves in nitric acid with a purple-red color, but soon turns yellow. It binds to a large number of metal ions such as Cr 3+ , Al 3+ or Fe 3+ / Fe 2+ . In aqueous solutions with aluminum ions (as mordant ) hematein exists in three forms, as yellow free hematein, with one aluminum ion as red hematein and with two aluminum ions as blue-violet hematein. Hematein is generated by the oxidation of hematoxylin, either by oxidation for three months with atmospheric oxygen (Ehrlich's hematoxylin or Delafield's hematoxylin), with sodium iodate (Mayer's hematoxylin) or with mercury oxide (Harris' hematoxylin). Both the binding of aluminum ions and the increase in pH produce a bathochromic color change from an absorption maximum at a wavelength of 445 nm to 560 nm.
Individual evidence
- ↑ a b Datasheet Hematein, for microscopy (Hist.) At Sigma-Aldrich , accessed on November 4, 2017 ( PDF ).
- ↑ Kazuko Shirai, Masaru Matsuoka: Structure and properties of hematein derivatives . In: Dyes and Pigments (1996), Volume 32, Issue 3, pp. 159-169. doi: 0143-7208 (96) 00022-8 .
- ↑ H. Puchtler, SN Meloan, FS Waldrop: Application of current chemical concepts to metal-hematein and -brazilein stains. In: Histochemistry. Volume 85, Number 5, 1986, pp. 353-364, PMID 2430916 .
- ↑ C. Bettinger, HW Zimmermann: New investigations on hematoxylin, hematein, and hematein-aluminum complexes. II. Hematein-aluminum complexes and hemalum staining. In: Histochemistry. Volume 96, Number 3, 1991, pp. 215-228, PMID 1717413 .
- ^ Carl Löwig: Chemistry of organic compounds . F. Vieweg, 1846, p. 867 .
- ↑ Anil B. Amin, Liisa Mortensen, Trygve T. Poppe: Histology Atlas, Normal Structure of Salmonids A Color Atlas, English, German, French and Spanish Legends . Histology atlas: salmonids, 1992, ISBN 978-82-992406-1-1 , p. 176 .
- ↑ Gary Gill: Cytopreparation. Springer Science & Business Media, 2012, ISBN 978-1-461-44933-1 , p. 165.
- ↑ John D. Bancroft: Theory and Practice of Histological Techniques. Elsevier Health Sciences, 2008, ISBN 978-0-443-10279-0 , p. 121.
- ↑ Catherine E. Housecroft: Chemistry. Pearson Education, 2010, ISBN 978-0-273-73308-9 , p. 440.