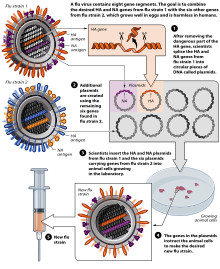
Influenza vaccine

An influenza vaccine (synonymous flu vaccine ) is a vaccine against the influenza virus . Influenza vaccines are used for flu vaccination (influenza vaccination).
properties
Influenza vaccines are purified antigens , split vaccines , inactivated viruses, or attenuated viruses . Due to the comparatively high genetic variability due to antigen shift and antigen drift (as mechanisms of immune evasion ) of the influenza viruses, the epitopes that are effective in a vaccine are often not present in the influenza viruses of the following season. As a result, the effectiveness of the approved influenza vaccines is limited to viruses similar to the vaccine strain and there is little immunity to other strains of influenza. Furthermore, the Paul Ehrlich Institute publishes current reference strains for seasonal influenza vaccines in Germany every year in accordance with the current recommendations of the World Health Organization and the Committee for Human Medicinal Products at the European Medicines Agency, on the basis of which the vaccine production is adapted. Due to the variability, these recommendations change almost every year for one or more strains. The reference strains include two influenza A viruses and one influenza B virus for common trivalent (trivalent) vaccines and a second influenza B virus for tetravalent (quadrivalent, tetravalent) vaccines. In contrast, pandemic influenza vaccines such as those against a highly pathogenic variant of the influenza A virus H5N1 , as the trigger of the avian flu H5N1 , or against the strain A / California / 7/2009 (H1N1) pdm09 of the influenza A virus H1N1 , as the trigger Pandemic H1N1 2009/10 , only one vaccine strain. Since the originally pandemic H1N1 virus strains have been circulating worldwide as seasonal flu since 2010 (with the usual variability), they have been among the reference strains for seasonal influenza vaccines since 2010. Influenza vaccines are on the World Health Organization's list of Essential Medicines .
immunology
Influenza vaccines produce neutralizing antibodies that prevent cells from re-infecting with the same strain of virus. To a small extent, these antibodies are cross-reactive with other influenza strains. The antibodies are mainly formed against the humoral immunodominant hemagglutinin and neuraminidase . These antibodies can be used in the course of a virological diagnosis to determine the titer in the vaccinated person or the vaccine serotype . Purified antigens, split or inactivated influenza vaccines are not absorbed into cells, which is why there is no pronounced cellular immune response .
Side effects
Adverse drug reactions from influenza vaccines include pain and swelling at the injection site and a day-long fever. The composition of the swine flu vaccine Pandemrix administered from 2009 to 2010 can presumably lead to narcolepsy in rare cases .
Manufacture of the vaccine
Culture media
The viruses for the flu vaccination are cultivated and multiplied in different media:
Chicken eggs
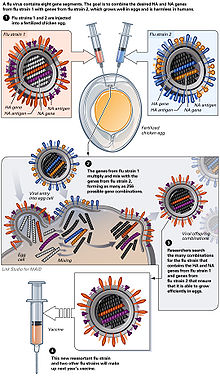
The virus is currently reproduced mainly in special incubated chicken eggs , the “specific pathogen free eggs”, which are 10–11 days old. In February of each year, the WHO decides on the composition of the seasonal winter vaccine. The so-called “seed virus” of the selected virus strains is sent to the manufacturer. In order to obtain optimal yields, the manufacturer carries out an HG (high-growth) reassortment . This takes about six weeks. The influenza virus multiplies in the Chorio - allantoic membrane. The eggs inoculated (inoculated) with the influenza virus are incubated for three days at 32 ° C. During this period, the virus multiplies very strongly. The eggs are opened and 6-7 ml of virus-containing allantoic fluid is harvested per egg. However, this type of production has disadvantages: The production of the vaccine takes about 6 months, the finished vaccine is available in June / July and is subjected to annual clinical studies. In the event of an influenza pandemic , large-scale egg production cannot be achieved due to the need for millions of eggs, as the planning of the logistical capacities required for this requires around 2 years in advance. In addition, egg vaccines require complicated purification and cause side effects, with egg protein allergies being a particular problem. Another shortcoming is the susceptibility of the production process to contamination and the necessary use of large quantities of antibiotics . Pandemic influenza strains are also very aggressive; In particular, strains of avian origin cannot be reproduced on chicken embryos.
Cell cultures
An alternative is to produce the vaccine in Vero cells . The advantages of this technology lie in the shortness of the production process (by eliminating the HG reassortment ) and the large production capacities. This enables a quick response to rapidly increasing demand. Vero cells are cultivated on an industrial scale in bioreactors with a capacity of several 1000 liters. Pandemic strains can be propagated with high yields. The sterile technology (technology from the point of view of the sterilisability and cleanability of the systems, as well as the retention capacity against microorganisms or biologically active substances) enables a safe design of the production facility. Dealing with pandemic influenza strains requires biological protection level 3 (BSL-3; Bio safety level), which cannot be achieved for egg facilities due to the process sequence (difficult to automate). The application made by the industry available flu vaccine just covers the average starting annual expected consumption, so the WHO on 19 August 2005 in the case of a renewed pandemic brought serious concerns about a looming shortage expressed.
Experimental procedure
Research is being carried out, for example, on the manufacture of the flu vaccine with the help of ciliates . The method should be faster and less risky than the previous standard.
Dead and live vaccines
Depending on the type of further vaccine preparation, a distinction is made between dead vaccines and live vaccines for influenza vaccines, as is the case with vaccines against other pathogens .
Dead vaccines
They are divided into:
- Inactivated whole particle vaccines (also: full virus vaccine): Inactivation (killing) of viruses by means of chemical substances / substance combinations, e.g. B. formaldehyde , beta-propiolactone and psoralen . The virus envelope is retained.
- (Inactivated) partial particle vaccines (also: split virus vaccine): Destruction (splitting) of the virus envelope with detergents or strong organic solvents. The viruses can also be inactivated (killed) with chemical substances.
- Subunit vaccines : the surface is completely dissolved and specific components ( hemagglutinin and neuraminidase proteins) are cleaned out. Another possibility is to produce the subunits recombinantly . Subunit vaccines are only slightly immunogenic , but have few side effects.
Live vaccines
In the production of live vaccines against influenza viruses, viruses are used that are attenuated but still able to reproduce ("vital"): ( English Live Attenuated Influenza Vaccine , LAIV , "living attenuated influenza vaccines"). A distinction is made here between
- Cold-adapted strains : these strains are only able to multiply at temperatures around 25 ° C, which limits the virus to the upper respiratory tract. Due to the lack of replication in the lower airways, only mild symptoms develop, not complete influenza. An example is the tribe A / Leningrad / 134/47/57 (H2N2)
- temperature-sensitive strains : the replication of these strains is limited to a temperature range of 38–39 ° C; here, too, the lower respiratory tract is not affected.
These vaccines are given intranasally . Their advantage over the previously used dead vaccines is that the vital viruses stimulate the immune system for longer and not only cause a humoral immune response , but also a cellular immune response . The disadvantage of influenza vaccines from pathogens that are still capable of reproducing is that the side effects are more frequent or more severe. The Swiss approval process for an attenuated influenza virus ( Nasaflu ) had to be canceled in 2001 due to paresis of the facial muscles. Therefore, attenuated influenza vaccines are not approved or recommended for pregnant women, people with a weakened immune system due to illness or under 2 or over 50 years of age.
Future influenza vaccines
The vaccines that have been approved to date target the bulges ("heads") of the hemagglutinin molecules on the surface of the virus. This molecule region changes in less than one influenza season due to antigen shift and antigen drift. This leads to fluctuating and on average only low effectiveness of the vaccines and the need to test them anew every year, to produce them in large quantities and to vaccinate them to all target persons. The health authorities of the USA and the European Union are therefore supporting the development of a “universal vaccine” against influenza, which, like the vaccines against almost all other pathogens, would only rarely need to be adjusted and re-vaccinated, but would be sufficiently effective over several seasons. One approach to this are vaccines that induce broadly neutralizing anti-IAV antibodies against the extracellular domain of matrix protein 2 (M2e) or against a certain area of hemagglutinin ( English stalk region , "stem region" between head and transmembrane domain ). Both the extracellular domain of M2 and the stem region of hemagglutinin are not very variable (highly conserved ) between the subtypes of influenza viruses . Therefore, a high selection pressure to preserve these amino acid sequences and their function is assumed, although the possibilities for immune evasion of the influenza viruses are limited as a result. Vaccines that attach to the stalk region of the hemagglutinin have been investigated on test subjects in Phase III since 2018 . Further approaches to increasing the broader effectiveness include the induction of cytotoxic T cells and T helper cells against conserved epitopes of several strains of the influenza viruses or their consensus sequence with the help of vectors , since cytotoxic T cells are only induced to a small extent by the split vaccines. Another approach is influenza vaccines made from messenger RNA , in the European Union for example supported by CORDIS and the UniVax project by institutions from seven EU countries. On the one hand, RNA vaccines are intended to ensure that the influenza virus no longer produces any reproductive offspring in the body of the vaccinated person. On the other hand, the immune system should be better prepared for future variants of the influenza virus.
WHO: recommended composition of vaccines since 1998/1999
For current recommendations, see flu vaccination
| Northern hemisphere of the earth | Southern hemisphere of the earth | ||
|---|---|---|---|
| season | composition | season | composition |
| 2020/2021 |
Chicken-based or live-attenuated influenza vaccines:
in the quadruple vaccine as a second B antigen:
cell-based influenza vaccines:
in the quadruple vaccine as a second B antigen:
|
2021 | |
| 2019/2020 | A / Brisbane / 02/2018 (H1N1), pdm09-like strain A / Kansas / 14/2017 (H3N2) -like strain B / Colorado / 06/2017-like strain (B / Victoria / 2/87 line) in the quadruple vaccine also variant of B / Phuket / 3073/2013-like strain (B / Yamagata / 16/88 line) |
2020 | A / Brisbane / 02/2018 (H1N1) pdm09-like strain A / South Australia / 34/2019 (H3N2) -like strain B / Washington / 02/2019-like strain (B / Victoria line) in the quadruple vaccine also B / Phuket / 3073/2013-like strain (B / Yamagata line) |
| 2018/2019 | A / Michigan / 45/2015 (H1N1) pdm09-like A / Singapore / INFIMH-16-0019 / 2016 (H3N2) -like B / Colorado / 06/2017-like (B / Victoria / 2/87-line) in quadruple vaccine also B / Phuket / 3073/2013-like (B / Yamagata / 16/88 line) |
2018 | A / Michigan / 45/2015 (H1N1) pdm09 A / Singapore / INFIMH-16-0019 / 2016 (H3N2) B / Phuket / 3073/2013 in the quadruple vaccine also B / Brisbane / 60/2008 |
| 2017/2018 | A / Michigan / 45/2015 (H1N1) pdm09 A / Hong Kong / 4801/2014 (H3N2) B / Brisbane / 60/2008 in the quadruple vaccine also B / Phuket / 3073/2013 |
2017 | A / Michigan / 45/2015 (H1N1) pdm09 A / Hong Kong / 4801/2014 (H3N2) B / Brisbane / 60/2008 in the quadruple vaccine also B / Phuket / 3073/2013 |
| 2016/2017 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Hong Kong / 4801/2014 (H3N2) B / Brisbane / 60/2008 in the quadruple vaccine also B / Phuket / 3073/2013 |
2016 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Hong Kong / 4801/2014 (H3N2) B / Brisbane / 60/2008 in the quadruple vaccine also B / Phuket / 3073/2013 |
| 2015/2016 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Switzerland / 9715293/2013 (H3N2) B / Phuket / 3073/2013 in the quadruple vaccine also B / Brisbane / 60/2008 |
2015 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Switzerland / 9715293/2013 (H3N2) (three similar ones are named) B / Phuket / 3073/2013 in the quadruple vaccine also B / Brisbane / 60/2008 |
| 2014/2015 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Texas / 50/2012 (H3N2) B / Massachusetts / 2/2012 |
2014 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Texas / 50/2012 (H3N2) B / Massachusetts / 2/2012 |
| 2013/2014 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Victoria / 361/2011 (H3N2) B / Massachusetts / 2/2012 |
2013 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Victoria / 361/2011 (H3N2) B / Wisconsin / 1/2010 |
| 2012/2013 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Victoria / 361/2011 (H3N2) B / Wisconsin / 1/2010 |
2012 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Perth / 16/2009 (H3N2) B / Brisbane / 60/2008 |
| 2011/2012 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Perth / 16/2009 (H3N2) (strains like A / Wisconsin / 15/2009) B / Brisbane / 60/2008 |
2011 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Perth / 16/2009 (H3N2) B / Brisbane / 60/2008 |
| 2010/2011 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Perth / 16/2009 (H3N2) (strains like A / Wisconsin / 15/2009) B / Brisbane / 60/2008 |
2010 | A / California / 7/2009 (H1N1) pdm09 (so-called "swine flu") A / Perth / 16/2009 (H3N2) B / Brisbane / 60/2008 |
| 2009/2010 | A / Brisbane / 59/2007 (H1N1) A / Brisbane / 10/2007 (H3N2) B / Brisbane / 60/2008 |
2009 | A / Brisbane / 59/2007 (H1N1) (strains like A / South Dakota / 6/2007) A / Brisbane / 10/2007 (H3N2) (like A / Brisbane / 10/2007 or A / Uruguay / 716/2007) B / Florida / 4/2006 (tribes like B / Brisbane / 3/2007) |
| 2008/2009 | A / Brisbane / 59/2007 (H1N1) A / Brisbane / 10/2007 (H3N2) B / Florida / 4/2006 (B / Brisbane / 3/2007) |
2008 | A / Solomon Islands / 3/2006 (H1N1) A / Brisbane / 10/2007 (H3N2) B / Florida / 4/2006 |
| 2007/2008 | A / Solomon Islands / 3/2006 (H1N1) A / Wisconsin / 67/2005 (H3N2) (like A / Hiroshima / 52/2005) B / Malaysia / 2506/2004 |
2007 | A / New Caledonia / 20/99 (H1N1) A / Wisconsin / 67/2005 (H3N2) B / Malaysia / 2506/2004 |
| 2006/2007 | A / New Caledonia / 20/99 (H1N1) A / Wisconsin / 67/2005 (H3N2) (A / Wisconsin / 67/2005 or A / Hiroshima / 52/2005) B / Malaysia / 2506/2004 (like B / Ohio / 1/2005 or B / Victoria / 2/87) |
2006 | A / New Caledonia / 20/99 (H1N1) A / California / 7/2004 (H3N2) (also A / New York / 55/2004) B / Malaysia / 2506/2004 |
| 2005/2006 | A / New Caledonia / 20/1999 (H1N1) A / California / 7/2004 (H3N2) (like A / New York / 55/2004) B / Jiangsu / 10/2003 |
2005 | A / New Caledonia / 20/99 (H1N1) A / Wellington / 1/2004 (H3N2) B / Shanghai / 361/2002 (B / Shanghai / 361/2002, B / Jilin / 20/2003 or B / Jiangsu / 10 / 2003) |
| 2004/2005 | A / New Caledonia / 20/99 (H1N1) A / Fujian / 411/2002 (H3N2) (like A / Wyoming / 3/2003 or A / Kumamoto / 102/2002) B / Shanghai / 361/2002 (like the tribes B / Shanghai / 361/2002 or B / Jilin / 20/2003). Because of the widespread use, B / Jiangsu / 10/2003 strains were also used. |
2004 | A / New Caledonia / 20/99 (H1N1) A / Fujian / 411/2002 (H3N2) (A / Kumamoto / 102/2002 and A / Wyoming / 3/2003 were similar virus isolates grown in chicken eggs) B / Hong Kong / 330 / 2001 (B / Shandong / 7/97, B / Hong Kong / 330/2001 and B / Hong Kong / 1434/2002; B / Brisbane / 32/2002 was also available) |
| 2003/2004 | A / New Caledonia / 20/99 (H1N1) A / Moscow / 10/99 (H3N2) (strains like A / Panama / 2007/99) B / Hong Kong / 330/2001 (strains like B / Shandong / 7/97 , B / Hong Kong / 330/2001, B / Hong Kong / 1434/2002) |
2003 | A / New Caledonia / 20/99 (H1N1) A / Moscow / 10/99 (H3N2) (strains like A / Panama / 2007/99) B / Hong Kong / 330/2001 (strains like B / Shandong / 7/97 , B / Hong Kong / 330/2001, B / Hong Kong / 1434/2002) |
| 2002/2003 | A / New Caledonia / 20/99 (H1N1) A / Moscow / 10/99 (H3N2) (strains like A / Panama / 2007/99) B / Hong Kong / 330/2001 |
2002 | A / New Caledonia / 20/99 (H1N1) A / Moscow / 10/99 (H3N2) (strains like A / Panama / 2007/99) B / Sichuan / 379/99 (strains like B / Guangdong / 120/2000, B / Johannesburg / 5/99 or B / Victoria / 504/2000) |
| 2001/2002 | A / New Caledonia / 20/99 (H1N1) A / Moscow / 10/99 (H3N2) (strains like A / Panama / 2007/99) B / Sichuan / 379/99 (strains like B / Johannesburg / 5/99 and B / Victoria / 504/2000) |
2001 | A / Moscow / 10/99 (H3N2) (strains like A / Panama / 2007/99) A / New Caledonia / 20/99 (H1N1) B / Sichuan / 379/99 (strains like B / Guangdong / 120/2000, B / Johannesburg / 5/99 or B / Victoria / 504/2000) |
| 2000/2001 | A / Moscow / 10/99 (H3N2) (strains like A / Panama / 2007/99) A / New Caledonia / 20/99 (H1N1) B / Beijing / 184/93 |
2000 | A / Moscow / 10/99 (H3N2) A / New Caledonia / 20/99 (H1N1) B / Beijing / 184/93 (strains like B / Shangdong / 7/97) |
| 1999/2000 | A / Sydney / 5/97 (H3N2) A / Beijing / 262/95 (H1N1) B / Beijing / 184/93 (tribes like B / Shangdong / 7/97) |
1999 | A / Sydney / 5/97 (H3N2) A / Beijing / 262/95 (H1N1) B / Beijing / 184/93 (vaccines such as B / Harbin / 7/94) |
| 1998/1999 | A / Sydney / 5/97 (H3N2) A / Beijing / 262/95 (H1N1) B / Beijing / 184/93 (vaccines such as B / Harbin / 7/94) |
1998 | |
Vaccines approved in Germany
For currently approved vaccines, see flu vaccination
season 2017/2018
| vaccine | comment | Substance group | virulence | Minimum age of the vaccinated |
Maximum age of the vaccinated |
administration |
|---|---|---|---|---|---|---|
| Afluria 2017/2018 | Split vaccine | inactivated | 5 | - | intramuscular | |
| Begipal 2017/2018 | Surface antigen subunit vaccine | inactivated | 0.5 | - | intramuscular | |
| Fluad 2017/2018 | Surface antigen subunit vaccine | inactivated | 65 | - | intramuscular | |
| Fluarix | Split vaccine | inactivated | 0.5 | - | intramuscular | |
| Fluenz Tetra 2017/2018 | Influenza virus types A, H1N1 / A, H3N2 / B (Victoria lineage) / B (Yamagata lineage) | all | live attenuated | 2 | 17th | nasal |
| Flu vaccine CSL | Split vaccine | inactivated | 5 | - | intramuscular | |
| Influenza vaccine STADA N 2017/2018 | Surface antigen subunit vaccine | inactivated | 0.5 | - | intramuscular | |
| IDflu | Strength: 15 µg | Split vaccine | inactivated | 60 | - | intradermal |
| Influs split SSW | Split vaccine | inactivated | 0.5 | - | intramuscular | |
| Influsplit Tetra 2017/2018 | Split vaccine | inactivated | 3 | - | intramuscular | |
| Influvac 2017/2018 | Surface antigen subunit vaccine | inactivated | 0.5 | - | intramuscular | |
| Influvac Tetra | Surface antigen subunit vaccine | inactivated | 18th | - | intramuscularly or deep subcutaneously | |
| INTANZA | Strength: 15 µg | Split vaccine | inactivated | 60 | - | intradermal |
| Optaflu | produced in cell culture | Surface antigen subunit vaccine | inactivated | 18th | - | intramuscular |
| Vaxigrip 2017/2018 | Split vaccine | inactivated | 0.5 | - | intramuscularly, if necessary deep subcutaneously | |
| Vaxigrip Tetra 2017/2018 | Split vaccine | inactivated | 3 | - | intramuscularly, if necessary deep subcutaneously | |
| Xanaflu 2017/2018 | Surface antigen subunit vaccine | inactivated | 0.5 | - | intramuscular | |
| Xanaflu Tetra | Surface antigen subunit vaccine | inactivated | 18th | - | intramuscularly or deep subcutaneously |
For the 10 vaccines with the indication 2017/2018, the changes for the 2017/2018 season were already approved on August 24, 2017.
literature
- DM Knipe, Peter M. Howley , DE Griffin, (Eds.): Fields Virology. 5th edition. Lippincott Williams & Wilkins, Philadelphia 2007, ISBN 978-0-7817-6060-7 .
Individual evidence
- ↑ WHO Model List of Essential Medicines. (PDF) In: World Health Organization. October 2013, accessed April 22, 2014 .
- ↑ J. Wrammert, K. Smith, J. Miller, WA Langley, K. Kokko, C. Larsen, NY Zheng, I. Mays, L. Garman, C. Helms, J. James, GM Air, JD Capra, R Ahmed, PC Wilson: Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. In: Nature . Volume 453, number 7195, May 2008, pp. 667-671, doi: 10.1038 / nature06890 . PMID 18449194 , PMC 2515609 (free full text).
- ↑ X. Cheng, M. Eisenbraun, Q. Xu, H. Zhou, D. Kulkarni, K. Subbarao, G. Kemble, H. Jin: H5N1 vaccine-specific B cell responses in ferrets primed with live attenuated seasonal influenza vaccines. In: PLOS ONE . Volume 4, Number 2, 2009, Art. E4436, doi: 10.1371 / journal.pone.0004436 . PMID 19209231 , PMC 2635969 (free full text).
- ^ I. Leroux-Roels, G. Leroux-Roels: Current status and progress of prepandemic and pandemic influenza vaccine development. In: Expert review of vaccines. Volume 8, number 4, April 2009, pp. 401-423, doi: 10.1586 / erv.09.15 . PMID 19348557 .
- ↑ Z. Wang, S. Tobler, J. Roayaei, A. Eick: Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. In: JAMA . Volume 301, Number 9, March 2009, pp. 945-953, doi: 10.1001 / jama.2009.265 . PMID 19255113 .
- ↑ N. Feltelius, I. Persson, J. Ahlqvist-Rastad, M. Andersson, L. Arnheim-Dahlström, P. Bergman, F. Granath, C. Adori, T. Hökfelt, S. Kühlmann-Berenzon, P. Liljeström , M. Maeurer, T. Olsson, .. Örtqvist, M. Partinen, T. Salmonson, B. Zethe: A coordinated cross-disciplinary research initiative to address an increased incidence of narcolepsy following the 2009–2010 Pandemrix vaccination program in Sweden. In: Journal of internal medicine. Volume 278, number 4, October 2015, pp. 335–353, doi: 10.1111 / joim.12391 . PMID 26123389 .
- ↑ MC Sturkenboom: The narcolepsy-pandemic influenza story: can the truth ever be unraveled? In: Vaccine. Volume 33 Suppl 2, June 2015, pp. B6-B13, doi: 10.1016 / j.vaccine.2015.03.026 . PMID 26022571 .
- ↑ Weekly epidemiological record / Relevé épidémiologique hebdomadaire ; 19 August 2005, 80th year / 19 août 2005, 80e année. (PDF; 220 kB) WHO; No. 33, 2005, 80, 277-288
- ^ New flu vaccine from Münster . Antenne Münster, April 11, 2019; accessed on July 7, 2019
- ↑ am: Flu vaccine from ciliates. In: Pharmaceutical newspaper. August 22, 2013. Retrieved October 21, 2013 .
- Jump up ↑ Robert B. Belshe, Kathryn M. Edwards, Timo Vesikari, Steven V. Black, Robert E. Walker, Micki Hultquist, George Kemble, Edward M. Connor: Live Attenuated versus Inactivated Influenza Vaccine in Infants and Young Children. In: New England Journal of Medicine. 356, 2007, p. 685, doi: 10.1056 / NEJMoa065368 .
- ↑ Christopher S. Ambrose, Catherine Luke, Kathleen Coelingh: Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. In: Influenza and Other Respiratory Viruses. 2, 2008, p. 193, doi: 10.1111 / j.1750-2659.2008.00056.x . PMC 2710797 (free full text).
- ↑ T. Jefferson, A. Rivetti, C. Di Pietrantonj, V. Demicheli, E. Ferroni: Vaccines for preventing influenza in healthy children. In: The Cochrane Library 8: CD004879. 2012. doi: 10.1002 / 14651858.CD004879.pub4 . PMID 22895945 .
- ^ P. Sendi, R. Locher, B. Bucheli, M. Battegay: Intranasal influenza vaccine in a working population. In: Clinical Infectious Diseases . Volume 38, Number 7, April 2004, pp. 974-980, doi: 10.1086 / 386330 . PMID 15034829 .
- ↑ Ashley P. Taylor: First Universal Flu Vaccine to Enter Phase 3 Trial , online November 12, 2018, accessed June 11, 2019
- ↑ NIH begins first-in-human trial of a universal influenza vaccine candidate . NIH April 3, 2019; accessed on June 11, 2019
- ↑ L. Moise, F. Terry, M. Ardito, R. Tassone, H. Latimer, C. Boyle, WD Martin, AS De Groot: Universal H1N1 influenza vaccine development: identification of consensus class II hemagglutinin and neuraminidase epitopes derived from strains circulating between 1980 and 2011. In: Human vaccines & immunotherapeutics. Volume 9, number 7, July 2013, pp. 1598–1607, doi: 10.4161 / hv.25598 . PMID 23846304 .
- ↑ K. Xiang, G. Ying, Z. Yan, Y. Shanshan, Z. Lei, L. Hongjun, S. Maosheng: Progress on adenovirus-vectored universal influenza vaccines. In: Human vaccines & immunotherapeutics. Volume 11, number 5, 2015, pp. 1209-1222, doi: 10.1080 / 21645515.2015.1016674 . PMID 25876176 , PMC 4514376 (free full text).
- ↑ EU Commission: A “Universal” Influenza Vaccine through Synthetic, Dendritic Cell-Targeted, Self-Replicating RNA Vaccines , accessed June 10, 2019
- ↑ UniVax project: self-description
- ↑ Francesco Berlanda Scorza1, Norbert Pardi: New Kids on the Block: RNA-Based Influenza Virus Vaccines . April 1, 2018, PMC 6027361 (free full text)
- ↑ WHO | Recommended composition of influenza virus vaccines for use in the 2020 - 2021 northern hemisphere influenza season. Retrieved May 22, 2020 .
- ^ Paul Ehrlich Institute - Influenza Vaccines. Retrieved May 22, 2020 .
- ^ Paul Ehrlich Institute - Influenza Vaccines. Retrieved May 22, 2020 .
- ^ Judith Koch, Sabine Vygen-Bonnet, Ole Wichmann: Standing Vaccination Commission (STIKO). The most important changes at a glance. In: Deutsche Ärzteblatt. Volume 116, Issue 39, (27 September) 2019, pp. B 1418 - B 1421, here: p. B 1421 ( vaccine composition 2019/2020 ).
- ↑ Recommended composition of influenza virus vaccines for use in the 2019-2020 northern hemisphere influenza season. Update from March 21, 2019
- ↑ Recommended composition of influenza virus vaccines for use in the 2020 southern hemisphere influenza season , September 27, 2019
- ↑ Recommended composition of influenza virus vaccines for use in the 2018-2019 northern hemisphere influenza season. WHO, February 22, 2018, accessed March 24, 2018 .
- ↑ Recommended composition of influenza virus vaccines for use in the 2018 southern hemisphere influenza season .
- ↑ who.int
- ↑ Recommended composition of influenza virus vaccines for use in the 2017 southern hemisphere influenza season .
- ↑ Recommended composition of influenza virus vaccines for use in the 2016-2017 northern hemisphere influenza season .
- ↑ Recommended composition of influenza virus vaccines for use in the 2016 southern hemisphere influenza season .
- ↑ Recommended composition of influenza virus vaccines for use in the 2015-2016 northern hemisphere influenza season .
- ↑ WHO - Recommended composition of influenza virus vaccines for use in the 2015 southern hemisphere influenza season .
- ↑ WHO - Recommended composition of influenza virus vaccines for use in the 2014-2015 northern hemisphere influenza season .
- ^ WHO - Recommended composition of influenza virus vaccines for use in the 2014 southern hemisphere influenza season .
- ↑ WHO - Recommended composition of influenza virus vaccines for use in the 2013-14 northern hemisphere influenza season .
- ↑ WHO - Recommended composition of influenza virus vaccines for use in the 2013 southern hemisphere influenza season .
- ↑ WHO - Recommended composition of influenza virus vaccines for use in the 2012-2013 northern hemisphere influenza season .
- ^ WHO - Recommended composition of influenza virus vaccines for use in the 2012 southern hemisphere influenza season .
- ↑ Recommended composition of influenza virus vaccines for use in the 2011–2012 northern hemisphere influenza season . World Health Organization (English)
- ↑ WHO / Europe recommendations on influenza vaccination during the 2011/2012 winter season . (PDF; 96 kB) World Health Organization (WHO), October 2011 (English); WHO: Frequently asked questions: Recommended composition of influenza virus vaccines for use in the northern hemisphere in the 2011–2012 influenza season . (PDF; 24 kB) February 17, 2011 (English)
- ↑ who.int (PDF; 103 kB)
- ↑ who.int , phac-aspc.gc.ca (PDF; 204 kB)
- ↑ who.int (PDF; 318 kB)
- ↑ who.int ,
- ↑ Centers for Disease Control and Prevention - Influenza (Flu) - Weekly Report: Influenza Summary Update 20, 2004-2005 Season .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ↑ who.int (PDF; 217 kB)
- ↑ who.int ,
- ↑ Update: Influenza Activity - United States and Worldwide, 2003-04 Season, and Composition of the 2004-05 Influenza Vaccine .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ↑ http://www.who.int/csr/disease/influenza/recommendations2004/en/index.html ( Memento from May 30, 2004 in the Internet Archive )
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ^ WHO - Recommendations for Influenza Vaccine Composition .
- ↑ Approved vaccines. ( Memento from June 26, 2019 in the Internet Archive ) PEI