Isopentyl acetate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
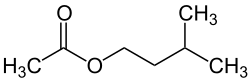
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isopentyl acetate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 14 O 2 | |||||||||||||||
| Brief description |
Not very volatile, colorless liquid with a fruity / banana-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 130.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.87 g cm −3 |
|||||||||||||||
| Melting point |
−79 ° C |
|||||||||||||||
| boiling point |
142 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
slightly soluble in water (2.12 g l −1 at 19.4 ° C) |
|||||||||||||||
| Refractive index |
1,400 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Isopentyl acetate is a chemical compound from the group of carboxylic acid esters , more precisely pentyl acetate .
Occurrence
Isopentyl acetate is one of the main components of banana flavor and an important flavoring agent in beer . It has also been detected as a flavoring substance in a variety of other fruits and other foods, especially fermented drinks. Bees release the compound before an attack, so it's an alarm pheromone.
Extraction and presentation
Isopentyl acetate ( 2 ) can be obtained by esterifying isoamyl alcohol ( 1 ) with acetic acid:
This acid-catalyzed equilibrium reaction can be shifted to the side of the ester 2 in that the water formed is removed from the reaction mixture.
properties
Isopentyl acetate is a slightly volatile, flammable, colorless liquid with a fruity odor that is sparingly soluble in water.
Safety-related parameters
Isopentyl acetate forms highly flammable vapor-air mixtures. The compound has a flash point of 35 ° C. The explosion range is between 1% by volume (53 g / m 3 ) as the lower explosion limit (LEL) and 9% by volume (485 g / m 3 ) as the upper explosion limit (UEL). The lower explosion point is 33 ° C. The ignition temperature is 380 ° C. The substance therefore falls into temperature class T2.
use
Isopentyl acetate is used as a banana aroma because of its banana smell. In the past, the Hefner candle was used as a light standard, using iso-amyl acetate = isopentyl acetate or a mixture of pentyl ester of acetic acid as fuel. In addition, because of its strong smell, it is also used for leak testing of military gas protective masks.
Individual evidence
- ↑ Entry on ISOAMYL ACETATE in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b c d e f g h i j k l m n o p q r Entry for CAS no. 123-92-2 in the GESTIS substance database of the IFA , accessed on September 21, 2018(JavaScript required) .
- ↑ Data sheet Isoamyl acetate, natural, ≥97%, FCC, FG from Sigma-Aldrich , accessed on September 20, 2015 ( PDF ).
- ^ Marshall J. Myers, Phillip Issenberg, Emily L. Wick: l-Leucine as a precursor of isoamyl alcohol and isoamyl acetate, volatile aroma constituents of banana fruit discs. In: Phytochemistry. 9, 1970, p. 1693, doi : 10.1016 / S0031-9422 (00) 85580-6 .
- ↑ Prof. Dr. Werner Back: Microbiology of Food Volume 5: Beverages . Behr's Verlag DE, 2008, ISBN 978-3-89947-956-0 , p. 144 ( limited preview in Google Book search).
- ↑ Entry on isopentyl acetate. In: Römpp Online . Georg Thieme Verlag, accessed on September 21, 2015.
- ↑ a b Jeff Potter: Cooking for Geeks Inspiration & Innovation for the Kitchen; [Recipes and Scientific Adventures] . O'Reilly Germany, 2011, ISBN 978-3-86899-125-3 , pp. 96 ( limited preview in Google Book search).
- ^ Hermann Sahm, Garabed Antranikian, Klaus-Peter Stahmann, Ralf Takors: Industrial microbiology . Springer-Verlag, 2014, ISBN 978-3-8274-3040-3 , pp. 265 ( limited preview in Google Book search).
- ^ A b E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ H. Lux, Modern Lighting, accessed on November 28, 2018.
- ^ Occupational Safety and Health Administration: Fit Testing Procedures (Mandatory). - 1910.134 App A | Occupational Safety and Health Administration , accessed January 17, 2020.

