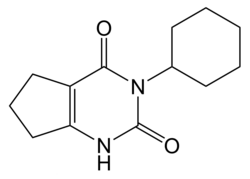
Lenacil
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Lenacil | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 13 H 18 N 2 O 2 | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 234.30 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.32 g cm −3 |
|||||||||||||||
| Melting point |
315.6-316.8 ° C |
|||||||||||||||
| Vapor pressure |
1 · 10 −9 hPa (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lenacil is an active ingredient for crop protection and the common name of a chemical compound from the group of uracil derivatives .
presentation
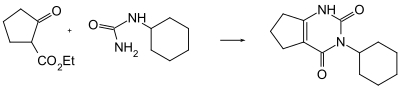
Lenacil can be obtained by a condensation reaction of ethyl 2-oxocyclopentane-1-carboxylate and cyclohexylurea with phosphoric acid.
properties
Lenacil is a flammable, colorless and odorless solid that is practically insoluble in water. It decomposes before it reaches its boiling point. It is largely stable under acidic and alkaline conditions.
use
Lenacil is used as an active ingredient in crop protection products. It is a selective herbicide for pre-emergence treatment, which is absorbed through the roots and acts by inhibiting photosynthesis . It is mainly used against seed weeds and annual grasses in spinach, sugar beet, strawberries and ornamental plants. It is distributed by DuPont .
Admission
In many EU countries, including Germany and Austria, as well as Switzerland, plant protection products containing this active ingredient are approved.
Web links
- Entry to lenacil in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on August 1, 2013.
- EU: Conclusion on the peer review of the pesticide risk assessment of the active substance lenacil. In: EFSA Journal. 7, 2009, p. 1326, doi : 10.2903 / j.efsa.2009.1326 .
Individual evidence
- ↑ a b c d e f g h i Entry on Lenacil in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c M. Bahadir, H. Parlar, Michael Spiteller: Springer Umweltlexikon . Springer, 2000, ISBN 978-3-540-63561-1 ( page 702 in the Google book search).
- ↑ a b Ullmann's Agrochemicals, Volume 1 . Wiley-VCH, 2007, ISBN 978-3-527-31604-5 ( page 809 in the Google book search).
- ↑ Entry on Lenacil in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Lenacil data sheet at Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 ( page 569 in the Google book search).
- ^ Terence Robert Roberts, DH Hutson: Metabolic pathways of agrochemicals, Volume 2 . Royal Soc of Chemistry, 1999, ISBN 978-0-85404-499-3 ( page 699 in Google book search).
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on Lenacil in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 13, 2016.