Linopirdine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
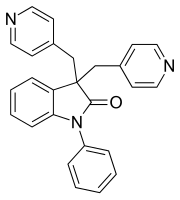
|
|||||||||||||
| General | |||||||||||||
| Surname | Linopirdine | ||||||||||||
| other names |
1-phenyl-3,3-bis (pyridin-4-ylmethyl) -1,3-dihydro-2 H -indol-2-one |
||||||||||||
| Molecular formula | C 26 H 21 N 3 O | ||||||||||||
| Brief description |
whitish solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 391.46 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
184-186 ° C |
||||||||||||
| solubility |
240 mg / mL in ethanol |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Linopirdine (DuP996) is a molecule developed by the American company DuPont and presented in 1990. It increases the release of neurotransmitters and directly blocks voltage-dependent potassium channels . It is a representative of the nootropics , a class of active ingredients that have a beneficial effect on the central nervous system , and has been investigated - albeit unsuccessfully - as a possible active ingredient against Alzheimer's disease .
effect
Linopirdine blocks various ion channels in nerve cells depending on the concentration , increases the duration of the action potential and synaptically increases the release of neurotransmitters , in particular acetylcholine and glutamate . In vivo it improves learning and memory in rats . It is also speculated that linopirdine can compensate for age-related attenuation of dopamine release in rats.
Applications
Linopirdine was tested under the name Aviva up to phase III for the treatment of Alzheimer's disease, but the effect of the substance has not been confirmed in double-blind studies . An improvement in memory in humans was also not observed. Structurally similar molecules, such as XE991, could be used as an active ingredient. Linopirdine is currently being investigated as an active ingredient in the treatment of tinnitus . In basic research, linopirdine is used as a selective potassium channel blocker.
Synthesis and structure
One method of synthesis is based on diphenylamine (1), which reacts with oxalyl chloride to form an amide (2). By ring closure, N-phenylisatin (3) is formed from it, which is then reacted with 4-picoline (4). The hydroxy group is esterified with acetic anhydride . The result is an acetic acid ester, from which an alkene is formed through elimination of acetic acid and then after hydrogenation (5). The 3-position of this product is sterically hindered , but at the same time it is activated by the adjacent C = O group and the benzene ring , so that a selective alkylation at this position with 4-chloromethylpyridine to linopirdine still occurs. The end product can be purified by recrystallization from 2-propanol .
Linopirdine and other nootropics
Modulation of synaptic connections is a generic characteristic of some nootropics. Thus, donepezil , rivastigmine and galantamine acetylcholinesterase inhibitors and memantine is an NMDA - antagonist . Modafinil's mechanism of action is not yet fully understood. Many of these substances are used for the symptomatic treatment of dementia .
Individual evidence
- ↑ a b c d e Datasheet Linopirdin from Sigma-Aldrich , accessed on October 18, 2016 ( PDF ).
- ↑ Tocris: Linopirdine , accessed November 26, 2015.
- ↑ VS Tam, M. Myers, L. Cook: Dup 996 (3,3-bis (pyrindinylmethyl) -1-phenyl-2-one) enhances the stimulus-induced release of acetylcholine from rat brain in vitro and in vivo. In: Drug Development Research Volume 19, Number 3, 1990, pp. 285-300. doi : 10.1002 / gdr.430190307
- ↑ SP Aiken, R. Zaczek, BS Brown: Pharmacology of the neurotransmitter release enhancer linopirdine (DuP 996), and insights into its mechanism of action. In: Advances in pharmacology (San Diego, Calif.). Volume 35, 1996, pp. 349-384, PMID 8920211 (review).
- ↑ ME Schnee, BS Brown: Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. In: The Journal of pharmacology and experimental therapeutics. Volume 286, Number 2, August 1998, pp. 709-717, PMID 9694925 .
- ↑ DJ Fontana, GT Inouye, RM Johnson: Linopirdine (DuP 996) improves performance in several tests of learning and memory by modulation of cholinergic neurotransmission. In: Pharmacology, biochemistry, and behavior. Volume 49, Number 4, December 1994, pp. 1075-1082, PMID 7886078 .
- ↑ GW Dent, BL Rule, SW Tam, EB De Souza: Effects of the memory enhancer linopirdine (Dup 996) on cerebral glucose metabolism in naive and hypoxia-exposed rats. In: Brain research. Volume 620, Number 1, August 1993, pp. 7-15, PMID 8402201 .
- ↑ GW Dent, BL Rule, Y. Zhan, R. Grzanna: The acetylcholine release enhancer linopirdine induces Fos in neocortex of aged rats. In: Neurobiology of aging. Volume 22, Number 3, 2001 May-Jun, pp. 485-494, PMID 11378256 .
- ↑ SW Tam, R. Zaczek: Linopirdine. A depolarization-activated releaser of transmitters for treatment of dementia. In: Advances in Experimental Medicine and Biology . Volume 363, 1995, pp. 47-56, PMID 7618529 .
- ↑ K. Rockwood, BL Beattie, MR Eastwood, H. Feldman, E. Mohr, W. Pryse-Phillips, S. Gauthier: A randomized, controlled trial of linopirdine in the treatment of Alzheimer's disease. In: The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. Volume 24, Number 2, May 1997, pp. 140-145, PMID 9164692 .
- ↑ A. Börjesson, T. Karlsson, R. Adolfsson, M. Rönnlund, L. Nilsson: Linopirdine (DUP 996): cholinergic treatment of older adults using successive and non-successive tests. In: Neuropsychobiology. Volume 40, Number 2, 1999, pp. 78-85, PMID 10474062 .
- ↑ R. Zaczek, RJ Chorvat, JA Saye, ME Pierdomenico, CM Maciag, AR Logue, BN Fisher, DH Rominger, RA Earl: Two new potent neurotransmitter release enhancers, 10,10-bis (4-pyridinylmethyl) -9 (10H ) -anthracenone and 10,10-bis (2-fluoro-4-pyridinylmethyl) -9 (10H) -anthracenone: comparison to linopirdine. In: The Journal of pharmacology and experimental therapeutics. Volume 285, Number 2, May 1998, pp. 724-730, PMID 9580619 .
- ↑ C. Wu, K. Gopal, GW Gross, TJ Lukas, EJ Moore: An in vitro model for testing drugs to treat tinnitus. In: European journal of pharmacology. Volume 667, number 1-3, September 2011, pp. 188-194, doi : 10.1016 / j.ejphar.2011.05.070 , PMID 21718695 .
- ^ WM Bryant, GF Huhn, JH Jensen, ME Pierce, C. Stammbach: A Large Scale Preparation of the Cognitive Enhancer Linopirdine. In: Synthetic Communications. Volume 23, number 11, 1993, pp. 1617-1615, doi : 10.1080 / 00397919308011258
- ↑ Lily C. Tang, Stephen J. Tang: Neurochemistry in Clinical Application . Springer Science & Business Media, 6 December 2012, ISBN 978-1-4615-1857-0 , p. 48–.
