Lithium iron phosphate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
||||||||||||||||
| General | ||||||||||||||||
| Surname | Lithium iron phosphate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | LiFePO 4 | |||||||||||||||
| Brief description |
gray to black powder (commercial form) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 157.759 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.8 - 1.4 g cm −3 ( bulk density ) |
|||||||||||||||
| Melting point |
> 300 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lithium iron phosphate is an inorganic compound that is used in lithium iron phosphate batteries to store charge. It is a mixed phosphate of iron and lithium and is usually marketed as a carbon-containing gray to black powder.
designation
According to the rules of inorganic nomenclature, compounds with several cations are listed in alphabetical order; therefore the compound should actually be called iron lithium phosphate. But that is not common. The material or the batteries equipped with it are sometimes referred to by the abbreviation LFP, which is derived from the empirical formula LiFePO 4 .
Occurrence
Lithium iron phosphate also occurs naturally in the form of the rather rare mineral triphyline .
history
Lithium iron phosphate was first discovered in the form of the aforementioned mineral triphyline . This was found in 1834 by the German mineralogist Johann Nepomuk von Fuchs in the Bavarian Forest . He examined it and found that it contained iron, lithium and phosphate, and he also found manganese. He named the new mineral.
In 1997, a research group led by John B. Goodenough was the first to propose the use of lithium iron phosphate as a cathode material in lithium-ion batteries.
Extraction and presentation
The starting materials for the preparation of lithium iron phosphate are lithium carbonate , lithium hydroxide or lithium phosphate , as well as iron salts such as iron carbonate , iron sulfate or iron phosphate . An example of such an implementation is the reaction
- .
Due to the increasing technical importance of LiFePO 4 , many different manufacturing processes have been developed: Solid-state syntheses with a calcination step at 400-800 ° C, often supplemented with grinding in the ball mill for better mixing, hydrothermal processes in which aqueous solutions are used under high pressure so that Temperatures above 100 ° C can be reached, and sol-gel processes . If the synthesis temperatures are high enough, the cheaper iron (III) salts can also be used, since Fe 3+ can be reduced to Fe 2+ by carbon in the heat (carbothermal reduction), e.g. B .:
- or
- .
Lithium iron phosphate is produced on an industrial scale, including by Phostech Lithium, which became a subsidiary of Süd-Chemie , which in turn was taken over by Clariant in 2011 . Phostech Lithium can produce 2500 tons of LiFePO 4 per year at its Candiac site in the Canadian province of Québec alone . In 2014, BASF put a plant in Weimar into operation with a production capacity of 3000 tons of LFP per year.
properties
Physical Properties
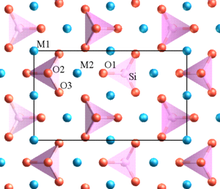
The electrical conductivity of LiFePO 4 is very low.
Lithium iron phosphate crystallizes in the olivine structure .
At low temperatures below 50 K, LiFePO 4 is antiferromagnetic .
Chemical properties
Lithium iron phosphate is soluble in hydrochloric acid. Lithium can be extracted from lithium iron phosphate while retaining the crystal lattice, which produces iron (III) phosphate FePO 4 .
LiFePO 4 is thermodynamically very stable; unlike lithium cobalt oxide , it does not give off any oxygen when heated .
use
Lithium iron phosphate is the lithium storage material (cathode material) at the positive pole of lithium iron phosphate batteries . When the battery is charged , iron (III) phosphate is formed , which is converted back into lithium iron (II) phosphate when it is discharged:
- Full Load: .
- complete discharge: .
The voltage of the lithium iron phosphate accumulators is 3.3 V somewhat lower than that of other lithium ion accumulators.
Due to the high stability of LiFePO 4 , batteries with this material are considered to be particularly safe. Therefore, the corresponding batteries are also used in electric vehicles , e.g. B. used in electric bicycles . Lithium iron phosphate is also used in some electric cars , e.g. B. in BYD e6 .
safety instructions
Lithium iron phosphate is considered non-toxic and therefore environmentally friendly.
literature
- Gouri Cheruvally: Lithium Iron Phosphate: A Promising Cathode-Active Material for Lithium Secondary Batteries . Trans Tech Publications Ltd, 2008, ISBN 978-0-87849-477-4 (126 pages).
- Pier Paolo Prosini: Iron Phosphate Materials as Cathodes for Lithium Batteries - The Use of Environmentally Friendly Iron in Lithium Batteries . Springer, London 2011, ISBN 978-0-85729-744-0 .
Individual evidence
- ↑ a b c d data sheet Lithium iron (II) phosphate, powder, <5 μm particle size (BET),> 97% (XRF) from Sigma-Aldrich , accessed on February 4, 2014 ( PDF ).
- ↑ Jian Wang, Yuan Chen, Lu Qi: The Development of Silicon Nanocomposite Materials for Li-Ion Secondary Batteries
- ↑ Johann Nepomuk von Fuchs: About a new mineral (triphyline) . In: Journal for Practical Chemistry . tape 3 , no. 1 , 1834, p. 98-104 , doi : 10.1002 / prac.18340030120 .
- ↑ Johann Nepomuk von Fuchs: Mixed notes, 3. Triphylin . In: Journal for Practical Chemistry . tape 5 , no. 1 , 1835, p. 319-320 , doi : 10.1002 / prac.18350050138 .
- ↑ Akshaya K. Padhi, KS Nanjundaswamy, John B. Goodenough: Phospho-Olivines as Positive Electrode Materials for Rechargeable Lithium Batteries . In: Journal of the Electrochemical Society . tape 144 , no. 4 , 1997, p. 1188-1194 , doi : 10.1149 / 1.1837571 .
- ↑ : Dragana Jugović, Dragan Uskoković: A review of recent developments in the synthesis procedures of lithium iron phosphate powders . In: Journal of Power Sources . tape 190 , 2009, p. 538-544 , doi : 10.1016 / j.jpowsour.2009.01.074 .
- ↑ Jing Du, Ling-Bin Kong, Hong Liu, Jin-Bei Liu, Mao-Cheng Liu, Peng Zhang, Yong-Chun Luo, Long Kang: Template-free synthesis of porous – LiFePO4 / C nanocomposite for high power lithium-ion batteries . In: Electrochimica Acta . tape 123 , 2014, pp. 1-6 , doi : 10.1016 / j.electacta.2013.12.157 .
- ↑ Clariant Innovation Spotlight: Cathode material for batteries - the safe bridge to e-mobility. (No longer available online.) Clariant international ltd, archived from the original on February 21, 2014 ; Retrieved February 3, 2014 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Angelina Hofacker: BASF starts commercial production of LFP cathode material . Production. In: Springer Fachmedien Wiesbaden GmbH (Ed.): News from automotive and engine technology . May 22, 2014 ( springerprofessional.de [accessed November 2, 2014]).
- ↑ Matthias Bartmann: BASF announces the start of commercial production of LFP cathode materials in Germany . May 19, 2014 ( basf.com [accessed November 2, 2014]).
- ^ RP Santoro and RE Newnham: Antiferromagnetism in LiFePO4 . In: Acta Crystallographica . tape 22 , no. 3 , March 1967, p. 344-347 , doi : 10.1107 / S0365110X67000672 .




