Lithium borohydride
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
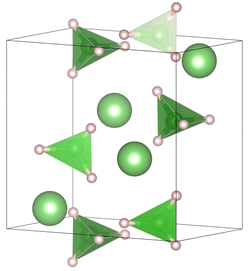
|
||||||||||||||||
| __ Li + __ H __ B 3+ | ||||||||||||||||
| Crystal system |
orthorhombic |
|||||||||||||||
| Space group |
Pnma (No. 62) |
|||||||||||||||
| Lattice parameters |
a = 7.17858 (4) Å, b = 4.43686 (2) Å, c = 6.80321 (4) Å |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Lithium borohydride | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | LiBH 4 | |||||||||||||||
| Brief description |
hygroscopic light gray solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 21.78 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.6660 g cm −3 |
|||||||||||||||
| Melting point |
280 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lithium borohydride is a chemical compound from the group of lithium compounds and boranes .
Extraction and presentation
Lithium borohydride can be produced by a metathesis reaction of sodium borohydride and lithium bromide .
The direct formation from metallic lithium , boron and hydrogen is possible in principle, but requires extreme conditions (150 at hydrogen pressure, 650 ° C).
Production from (intermediate) diborane (for example from lithium hydride with boron trifluoride in diethyl ether ) is also possible.
properties
Lithium borohydride is a flammable, hygroscopic, white to gray solid. It decomposes on contact with water or moist air and forms hydrogen in the process.
The compound crystallizes at room temperature in the space group Pnma (space group no. 62) with the lattice constants a = 7.17858 (4) Å, b = 4.43686 (2) Å, c = 6.80321 (4) Å in one Orthorhombic distorted wurtzite structure . At higher temperatures there is a hexagonal crystal structure in the space group P 6 3 mc (space group no.186 ) with the lattice constants a = 4.27631 (5) Å, c = 6.94844 (8) Å.
use
Lithium borohydride is used as a reducing agent for aldehydes , ketones , lactones , epoxides and esters in organic chemistry.
Individual evidence
- ↑ a b J-Ph. Soulie, G. Renaudin, R. Cerny, K. Yvon: Lithium borohydride LiBH 4 : I. Crystal structure . In: Journal of Alloys and Compounds . tape 346 , no. 1– ² , November 18, 2002, pp. 200-205 , doi : 10.1016 / S0925-8388 (02) 00521-2 .
- ↑ a b c d e f g h Entry on lithium borohydride in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 793.
- ↑ Peter Rittmeyer, Ulrich Wietelmann: hydride. In Ullmann's Encyclopedia of Industrial Chemistry . 2002, Wiley-VCH, Weinheim, doi : 10.1002 / 14356007.a13_199 .
- ↑ a b Patent DE10302262A1 : Process for the production of lithium borohydride.





