Meclozin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| ( S ) -form (top) and ( R ) -form (bottom), 1: 1 stereoisomeric mixture | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Meclozin | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula |
|
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
206–216 ° C with decomposition (racemate dihydrochloride) |
||||||||||||
| boiling point |
230 ° C |
||||||||||||
| pK s value |
3.1; 6.2 |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Meclozine is a drug selected from the group of systemic H 1 - antihistamines . It works against nausea and vomiting and experience has shown that it can also be used safely during pregnancy . However, it has not been on the market in Germany since 2007 (former trade name : Postadoxin N ® ). In other EU countries, preparations are or were known under the name Postafene or Agyrax .
synthesis
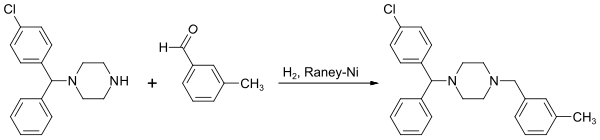
The synthesis of meclozine starts from (4-chlorophenyl) phenylmethanol, which is first converted to a 4-chlorophenylphenylpiperazinylmethane intermediate after halogenation by means of thionyl chloride and reaction with acetylpiperazine. After the acetyl group has been split off under acidic conditions, N -alkylation takes place on the piperazine ring with 3-methylbenzyl chloride to give the racemic target molecule.
Alternatively, the last synthesis step can be carried out as a reductive N -alkylation on the piperazine ring with 3-methylbenzaldehyde .
The racemate is formed during the synthesis . For pharmaceutical applications, the compound is converted into the dihydrochloride.
Clinical information
Meclozin is used for nausea and vomiting , especially for motion sickness and vomiting during pregnancy .
The safety for use during pregnancy has been well documented and it is even considered the drug of choice for vomiting. An alternative is doxylamine , among others .
Pharmacological properties
Meclozin works by competitive inhibition of histamine H 1 receptors . Since it crosses the blood-brain barrier as a first-generation antihistamine , it is not only antiemetic , but also makes you tired.
Stereoisomerism
Meclozin contains a stereogenic center and is chiral . The drug is used as a racemate {1: 1 mixture of ( R ) -1 - [(4-chlorophenyl) phenylmethyl] -4 - [(3-methylphenyl) methyl] piperazine and ( S ) -1 - [(4- Chlorophenyl) phenylmethyl] -4 - [(3-methylphenyl) methyl] piperazine} is used.
Individual evidence
- ↑ M. Brandstätter Kuhnert, R. Hoffmann, M. Senn: Thermo-Microscopic and Spectrophotometric Determination of Anti Histamines and Related Compounds , in: Microchem® J . , 1963 , 7 , pp. 357-374.
- ↑ a b c Entry on meclozin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Entry on meclozin. In: Römpp Online . Georg Thieme Verlag, accessed on January 14, 2019.
- ↑ a b Data sheet Meclizine dihydrochloride, ≥97% (HPLC) from Sigma-Aldrich , accessed on October 31, 2016 ( PDF ).
- ^ A b c A. Kleemann, J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition, Thieme 2001, ISBN 3-13-115134-X .
- ↑ J.-H. Fuhrkop, G. Li: Organic Synthesis - Concepts and Methods , Wiley-VCH 2003, p. 237, ISBN 978-3-527-30272-7 .
- ↑ US 2,709,169 (UCB, 1955).
- ↑ Källén B, Mottet I: Delivery outcome after the use of meclozine in early pregnancy . In: European Journal of Epidemiology . 18, No. 7, 2003, pp. 665-669. PMID 12952140 . Retrieved September 17, 2010.
- ↑ ANTIEMETIC THERAPY FOR PREGNANCY SUCCESS at arznei-telegramm.de .
- ↑ Embryotox: drug safety during pregnancy and lactation: Database drugs and active ingredients: Meclozin .