Methacrylonitrile
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
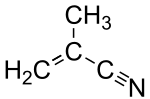
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methacrylonitrile | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 5 N | |||||||||||||||
| Brief description |
light-sensitive, volatile, colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 67.09 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.80 g cm −3 |
|||||||||||||||
| Melting point |
−36 ° C |
|||||||||||||||
| boiling point |
90 ° C |
|||||||||||||||
| Vapor pressure |
104 m bar (30 ° C) |
|||||||||||||||
| solubility |
little in water (25.7 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 1 ml m −3 or 3 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Methacrylonitrile is a chemical compound from the nitrile group . It is the nitrile of methacrylic acid , which is a colorless, highly flammable, poisonous and pungent-smelling liquid at room temperature.
Extraction and presentation
Methacrylonitrile can be obtained by reacting methacrylic acid or methacrylic acid methyl ester with ammonia under a catalytic gas phase reaction at temperatures of 250 ° C to 500 ° C. Alumina can be used as a catalyst .
properties
Methacrylonitrile is light-sensitive and at room temperature it is a colorless, volatile liquid with a pungent odor. This solidifies at −36 ° C and boils at 90 ° C. Methacrylonitrile has a flash point of −1 ° C and an ignition temperature of 465 ° C. The lower explosion limit of methacrylonitrile is 1.7% by volume and the upper explosion limit is 13.2% by volume. Methacrylonitrile falls into temperature class T1 due to its ignition temperature .
use
Methacrylonitrile is used in group transfer polymerization .
safety instructions
Methacrylonitrile is toxic and is mainly absorbed via the respiratory tract and the skin. After absorption, the substance has a toxic effect on the central and peripheral nervous system . Methacrylonitrile vapors can form explosive mixtures with air.
See also
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on methacrylonitrile in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on methacrylonitrile in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limits - Current MAK and BAT values (search for 126-98-7 or methacrylonitrile ), accessed on November 2, 2015.

