Methylpentynol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
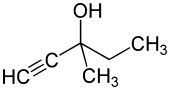
|
||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Methylpentynol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 10 O | |||||||||||||||
| Brief description |
colorless, odorless, burning-tasting liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 98.15 g · mol -1 | |||||||||||||||
| density |
0.87 g cm −3 |
|||||||||||||||
| Melting point |
−30 ° C |
|||||||||||||||
| boiling point |
121 ° C |
|||||||||||||||
| Vapor pressure |
7 h Pa (20 ° C) |
|||||||||||||||
| solubility |
easily in water (112 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.431 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Methylpentynol ( INN ), also methylpentinol or meparfynol , is a drug from the group of sedatives and hypnotics . Chemically, it is a combination of alkynes and alcohols that was patented by Bayer in 1913 .
The carbamate of methylpentynol was used as an active ingredient in sleeping pills and sedatives. Due to the development of substances with a more favorable action and safety profile, methylpentynol no longer has any therapeutic significance. In Germany, Austria and Switzerland there are no longer any preparations on the market.
Properties and safety information
Methylpentynol is a colorless, odorless, highly flammable liquid that dissolves easily in water. The mixtures of methylpentynol vapor with air are explosive in the range between 1.8 and 16% by volume. The substance is hazardous to water. In animal experiments with mice and rats, methylpentynol showed symptoms such as drowsiness, muscle twitching and reduced height gain, despite relatively high LD 50 values, even at lower doses.
use
By reaction with 2-butanone in liquid ammonia and in the presence of potassium hydroxide may be 3,6-dimethyl-4-octyne-3,6-diol are obtained.
Individual evidence
- ↑ Entry on methylpentynol. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c d e f g h Entry on 3-methyl-1-pentyn-3-ol in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Data sheet 3-Methyl-1-pentyn-3-ol from Sigma-Aldrich , accessed on November 7, 2016 ( PDF ).
- ^ A b Psychopharmacology Service Center, Bulletin. Vol. 2, Pg. 17, 1963 .
- ^ A b British Journal of Pharmacology and Chemotherapy . Vol. 11, Pg. 20, 1956 .
- ↑ Google Patents: EP1808426A1 - Process for the preparation of alkynediols - Google Patents , accessed December 4, 2018.