N -hydroxymaleimide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | N-hydroxymaleimide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 3 NO 3 | |||||||||||||||
| Brief description |
light yellow to brown crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 113.07 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
soluble in tetrahydrofuran , N , N -dimethylformamide , pyridine , methanol , ethanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
N -hydroxymaleimide is an unsaturated cyclic imide whose nitrogen atombearsa hydroxyl group . As in maleimide and in N -alkyl and N -aryl maleimides, the two carbonyl groups adjacent to the double bond havea strong electron-withdrawing effect, making N -hydroxymaleimide a very reactive dienophile in Diels-Alder reactions .
Manufacturing
Earlier syntheses of N -hydroxymaleimide also use the Diels-Alder reaction of the starting compound maleic anhydride with furan to form the Diels-Alder adduct exo-3,6-epoxy-1,2,3,6-tetrahydrophthalic anhydride to introduce a protective group for the activated double bond in maleic anhydride.
After the primary adduct has reacted with hydroxylamine , the reactive N - hydroxyl group is reacted with phenyl chloroformate and thus provided with the phenyloxycarbonyl protective group. First, furan is split off by heating at temperatures below 170 ° C. in a retro-Diels-Alder reaction , and with a further increase in temperature, the phenyloxycarbonyl group is also separated off and the target product N -hydroxymaleimide is obtained in low overall yields of about 12%. One variant uses the trimethylsilyl group from the reagent 1,2-bis (trimethylsilyl) urea as a protective group for the N -hydroxy group, which can be split off hydrolytically.
Neither publication provides any information about the purities obtained for the end product.
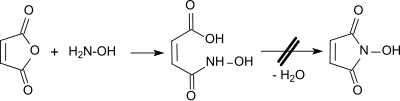
The obvious synthetic route to N -hydroxymaleimide, starting from maleic anhydride and hydroxylamine, leads in the first stage to maleic monohydroxamic acid, which is obtained with dehydrating agents such as. B. acetic anhydride or POCl 3 could not be cyclized to the N -hydroxymaleimide.
properties
N -hydroxymaleimide is obtained in the synthesis as a light yellow to brown solid, which is described as hygroscopic, corrosive and sensitive to air and moisture. After recrystallization from n- hexane - THF , it is obtained as a white, crystalline substance. The substance reacts acidic in aqueous solution, as the two carbonyl groups in the N -hydroxymaleimide molecule activate the hydrogen atom of the N -hydroxy group. The activation of the double bond in turn leads to easy addition of nucleophiles , such as. B. amino and especially thiol groups.
Applications
N -hydroxymaleimide was used as a dienophile for the synthesis of the first stage of new anxiolytics and antidepressants .
The tendency of N -hydroxymaleimide to Diels-Alder reactions with furans can also be used to build thermally reversibly crosslinked polymers , e.g. B. for the reversible encapsulation of electronic components.
The acidity of the N -hydroxy group can be used to build up crosslinked organosiloxanes, in which the addition of N -hydroxymaleimide slows down the crosslinking at room temperature.
The high acidity together with the pronounced thermal stability of the N -hydroxymaleimide structures make them interesting starting materials for polymeric photoresists with high resolution and the possibility of development with aqueous-alkaline media. However, N -hydroxymaleimide, as 1,2-disubstituted ethylene with an activated hydrogen on the N -hydroxy group, cannot be polymerized radically to give homopolymers with higher molar masses.
Of several protective groups on the acidic N -hydroxy group that have been investigated, the isopropyloxycarbonyl group in particular has proven itself. The protected N -Hydroxymaleimide can with AIBN in 1,4-dioxane solution radically to homopolymers and copolymers with z. B. styrene can be polymerized with useful molecular weights (M n > 10,000).
The isopropyloxycarbonyl protective group can be cleaved thermolytically at 205 ° C, resulting in poly- N- hydroxymaleimide, which dissolves in methanol and aqueous-alkaline media, but is insoluble in many organic solvents. Copolymers with the N -hydroxymaleimide monomer have extremely high glass transition temperatures of 240 ° C. and are soluble in basic media, which indicates their suitability as photoresist materials.
N -hydromaleimide is the starting material for the production of so-called polymeric N-hydroxysuccinimide , ie for obtaining solid carriers for active esters for peptide synthesis. One approach is based on chloromethylated polystyrene obtained by the Blanc reaction , which reacts with thiourea and subsequent alkaline hydrolysis of the isothiourea formed to give the corresponding thiol . The thiol group easily adds to the activated double bond of the N-hydroxymaleimide to form the H-hydroxysuccinimide, which is polymer-fixed via a thioether function.
A completely different route leads via the Diels-Alder precursor of N -hydroxymaleinimide (chemically correct: exo- N -hydroxy-7-oxabicyclo [2.2.1] hept-5-ene-2,3-dicarboximide), which is made with Carbodiimides, e.g. B. Diisopropylcarbodiimide can be activated for smooth reaction with carboxylic acids . The resulting active esters can be converted into insoluble polymers with Grubbs catalysts and a bifunctional crosslinker by ring-opening metathesis polymerization (ROMP), which react with primary and secondary amino compounds under mild conditions to form amides in high yields.
The polymeric N-hydroxysuccinimide can be recovered by simple filtration. This route also allows the production of polymer-fixed, activated Mosher esters from Mosher acid , with which chiral amines are converted into diastereomeric amides, on which the enantiomeric excess of stereospecific reactions can be determined without prior, laborious purification by means of 1H NMR spectroscopy .
Individual evidence
- ↑ a b c d data sheet N-hydroxymaleinimide from Sigma-Aldrich , accessed on May 28, 2015 ( PDF ).
- ↑ a b c d B. Bessières, N-Hydroxymaleinimide , e-EROS Encyclopedia of Reagents for Organic Synthesis , 2008, doi: 10.1002 / 047084289X.rn00820
- ↑ JV Staros: N-Hydroxysuccinimide active esters: Bis (N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant protein cross-linkers . In: Biochemistry . tape 21 , no. 17 , 1982, pp. 3950-3955 , doi : 10.1021 / bi00260a008 .
- ↑ a b D.P. Vanderbilt, Bifunctional synzymes via alternating copolymerization , Ph.D. Thesis, University of Florida, 1982
- ↑ a b Patent EP0123321 : Process for crosslinking organopolysiloxane compositions. Applied on April 26, 1984 , published on March 27, 1987 , applicant: Wacker-Chemie GmbH, inventor: U. Michel, J. Radecker.
- ↑ J. Kossakowski, M. Krawiecka: Synthesis of new N-substituiertem cyclic imide with at expected anxiolytic activity. Derivatives of N-Hydroxy-1-methoxybicyclo [2.2.2] oct-5-ene-2,3-dicarboximide . In: Acta Polon. Pharm., Drug Res. Band 60 , no. 3 , 2003, p. 177-182 ( PDF ).
- ↑ Patent US6271335B1 : Method of making thermally removable polymeric encapsulants. Filed January 18, 2000 , published August 7, 2001 , applicant: Sandia Corp., inventor: JH Small, DA Loy, DR Wheeler, JR McElhanon, RS Saunders.
- ↑ K.-D. Ahn, CG Wilson: Synthesis of polymers having N-hydroxymaleimide units by thermolysis of N- (isopropyloxycarbonyloxy) maleimide polymers . In: Bull. Korean Chem. Soc. tape 16 , 1995, pp. 443-449 ( PDF ).
- ↑ M. Adamczyk, JR Fishpaugh, PG Mattingly: Preparation and use of N-hydroxysuccinimidyl active ester resins . In: Tetrahedron Lett. tape 40 , no. 3 , 1999, p. 463-466 , doi : 10.1016 / S0040-4039 (98) 02425-3 .
- ↑ AGM Barrett, SM Cramp, RS Roberts, FJ Zécri: A ROMPGEL-Supported N-Hydroxysuccinimide: A Host of Acylations with Minimal Purification . In: Org. Lett. tape 2 , no. 2 , 2000, pp. 261-266 , doi : 10.1021 / ol991208w .
- ↑ GR Sullivan, JA Dale, HS Mosher: Correlation of configuration and fluorine-19 chemical shifts of α-methoxy-α-trifluoromethylphenyl acetate derivatives . In: J. Org. Chem. Band 38 , no. 12 , 1973, p. 2143-2147 , doi : 10.1021 / jo00952a006 .
- ↑ T. Arnauld, AGM Barrett, BT Hopkins, FJ Zécri: Facile and purification free synthesis of Mosher amides utilizing a ROMPgel supported reagent . In: Tetrahedron Lett. tape 42 , no. 46 , 2001, p. 8215-8217 , doi : 10.1016 / S0040-4039 (01) 01724-5 .