Nile red
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
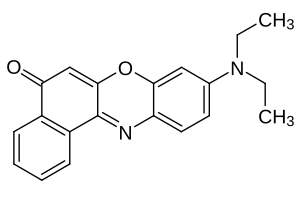
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Nile red | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 20 H 18 N 2 O 2 | ||||||||||||||||||
| Brief description |
dark green solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 318.37 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
203-205 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Nile red , as Nile blue oxazone referred to is a lipophilic fluorescent phenoxazine - dye .
Manufacturing
Nile red is made by boiling a solution of Nile blue with sulfuric acid . The amino group is replaced by a carbonyl group .
Properties and use

V. l. right: 1. water , 2. methanol , 3. ethanol , 4. acetonitrile , 5. dimethylformamide , 6. acetone , 7. ethyl acetate , 8. dichloromethane , 9. n- hexane , 10. tert -butyl methyl ether , 11. cyclohexane , 12. Toluene .
Nile red is a highly fluorescent laser dye . It fluoresces in the red range down to the near infrared (NIR) and has an emission maximum at around 650 nm . Both the emission maximum and the quantum yield are strongly dependent on the solvent used. In n- heptane the emission maximum is around 520 nm, in acetone, however, it is 600 nm. The fluorescence in acetone is around 80 times weaker. A higher polarity of the solvent generally leads to a red shift of the fluorescence maximum and to a decrease in the quantum yield and the fluorescence lifetime of Nile red.
In microbiology, nile red is for labeling and staining ( engl. Staining ) used by cells or cell constituents. The fluorescence depends on the hydrophobicity of the lipids. Polar, that is, more hydrophilic lipids, such as phospholipids, which are essentially located in the cell membrane , fluoresce red. Neutral fats, such as triglycerides or cholesterol esters in intracellular fat droplets, on the other hand, fluoresce at shorter wavelengths (towards yellow). The fluorescence can be measured both microscopically and by means of flow cytometry .
Nile red is used in biochemistry for staining proteins in the course of protein characterization, e.g. B. SDS-PAGE and isoelectric focusing .
Individual evidence
- ↑ a b c d data sheet Nile Red, suitable for fluorescence, BioReagent, ≥98.0% (HPLC) from Sigma-Aldrich , accessed on November 18, 2019 ( PDF ).
- ↑ H. Tajalli include: The photo physical properties of Nile red and Nile blue in ordered anisotropic media. In: Dyes and Pigments 78, 2008, pp. 15-24.
- ↑ JN Lampe et al.: Nile Red is a fluorescent allosteric substrate of cytochrome P450 3A4. In: Biochemistry 47, 2008, pp. 509-516. PMID 18092806
- ↑ SD Fowler et al: Use of nile red for the rapid in situ quantitation of lipids on thin-layer chromatograms. In: J Lipid Res 28, 1987, pp. 1225-1232. PMID 3681147
- ↑ a b P. Greenspan et al: Nile red: a selective fluorescent stain for intracellular lipid droplets. In: J Cell Biol 100, 1985, pp. 965-973. PMID 3972906 .
- ↑ M. Sutter et al .: Sensitive spectroscopic detection of large and denatured protein aggregates in solution by use of the fluorescent dye Nile red. In: Journal of Fluorescence 17, 2007, pp. 181-192. PMID 17294134 ; PMC 1915606 (free full text).
- ↑ G. Diaz et al.: Hydrophobic characterization of intracellular lipids in situ by Nile Red red / yellow emission ratio. In: Micron 39, 2008, pp. 819-824. PMID 18329888
- ↑ A. Bermudez, JR Daban, JR Garcia, E. Mendez: Direct blotting, sequencing and immunodetection of proteins after five-minute staining of SDS and SDS-treated IEF gels with Nile red. In: BioTechniques. Volume 16, Number 4, April 1994, pp. 621-624, PMID 8024781 .
- ↑ FJ Alba, S. Bartolomé, A. Bermúdez, JR Daban: Fluorescent labeling of proteins and its application to SDS-PAGE and western blotting. In: Methods in molecular biology (Clifton, NJ). Volume 536, 2009, pp. 407-416, doi : 10.1007 / 978-1-59745-542-8_41 . PMID 19378078 .
further reading
- A. Nath et al: Spectral resolution of a second binding site for Nile Red on cytochrome P4503A4. In: Arch Biochem Biophys 474, 2008, pp. 198-204. PMID 18395506 .
- A. Rei et al .: Nile Red Synchronous Scan Fluorescence Spectroscopy to Follow Matrix Modification in Sol-Gel Derived Media and its Effect on the Peroxidase Activity of cytochrome c. In: J Fluoresc 18, 2008, pp. 1083-1091. PMID 18365305 .
- G. Genicot et al: The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. In: Theriogenology 63, 2005, pp. 1181-1194. PMID 15710202 .
- E. Bonilla and A. Prelle: Application of nile blue and nile red, two fluorescent probes, for detection of lipid droplets in human skeletal muscle. In: J Histochem Cytochem 35, 1987, pp. 619-621. PMID 3559182 .
- JN Lampe et al: Nile Red is a fluorescent allosteric substrate of cytochrome P450 3A4. In: Biochemistry 47, 2008, pp. 509-516. PMID 18092806 .
- J. Han et al: Chemiluminescent energy-transfer cassettes based on fluorescein and nile red. In: Angew Chem Int Ed 46, 2007, pp. 1684-1687. PMID 17397078 .
- K. Tainaka et al .: Nile Red nucleoside: novel nucleoside analog with a fluorophore replacing the DNA base. In: Nucleic Acids Symp Ser 49, 2005, pp. 155-156. PMID 17150680 .
- S. Mukherjee et al .: Membrane localization and dynamics of Nile Red: effect of cholesterol. In: Biochim Biophys Acta 1768, 2007, pp. 59-66. PMID 16934217 .
- ML Ferrer and F. del Monte: Enhanced emission of nile red fluorescent nanoparticles embedded in hybrid sol-gel glasses. In: J Phys Chem B 109, 2005, pp. 80-86. PMID 16850987 .
- KJ Thomas et al: A long-wavelength fluorescent glucose biosensor based on bioconjugates of galactose / glucose binding protein and Nile Red derivatives. In: Diabetes Technol Ther 8, 2006, pp. 261-268. PMID 16800747 .
- K. Sebok-Nagy et al: Interaction of 2-hydroxy-substituted Nile red fluorescent probe with organic nitrogen compounds. In: Photochem Photobiol 81, 2005, pp. 1212-1218. PMID 15901209 .
- DL Sackett and J. Wolf: Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. In: Anal Biochem 167, 1987, pp. 228-234. PMID 3442318 .
- J. Jose and K. Burgess: Syntheses and properties of water-soluble Nile Red derivatives. In: J Org Chem 71, 2006, pp. 7835-7839. PMID 16995693 .
- A. Steinbüchel and others: Microbiological internship. Verlag Springer, 2003, ISBN 3-540-44383-5 , p. 384.
- A. Douhal: Cyclodextrin Materials Photochemistry, Photophysics and Photobiology. Verlag Elsevier, 2006, ISBN 0-444-52780-X , pp. 49-50.