Phellogenic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
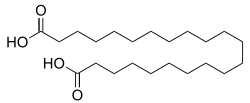
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phellogenic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 22 H 44 O 3 | |||||||||||||||
| Brief description |
colorless solid or white powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 370.57 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.9480 g cm −3 |
|||||||||||||||
| Melting point | ||||||||||||||||
| solubility |
almost insoluble in water, soluble in tetrahydrofuran and diethyl ether |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phellogenic acid or chemically 1,22-docosanedioic acid is a chemical compound from the group of α, ω- dicarboxylic acids .
Occurrence
Phellogenic acid occurs naturally in cork and Japanese wax .
Manufacturing
A standard process for the production of docosanedioic acid makes use of the general method of lengthening the chain length of carboxylic acids by six or of dicarboxylic acids by twelve carbon atoms by single or, in the case of dicarboxylic acids, double reaction of the corresponding acid chlorides with the cyclic enamine 1-morpholino-1-cyclohexene and then Wolff-Kishner reduction of the intermediate 7,16-diketodocosanoic acid.
[Similarly, 1-morpholino-1-cyclopentene can be used to extend the length by five or ten carbon atoms. ]
The other older synthetic routes to docosanedioic acid cited in the monograph on Organic Syntheses are complex and unproductive in terms of starting materials and process conditions. B. the mentioned reaction of α, ω-Diiodeicosan with potassium cyanide and hydrolysis of the dinitrile formed.
The best access to 1,22-docosanedioic acid is offered by the copper (I) chloride- catalyzed oxidative coupling of 10-undecinic acid to 10,12-docosadiyne-1,22-diacid with a yield of 90% of theory. and their practically quantitative catalytic hydrogenation to the title compound.
The starting compound 10-undecynoic is on from castor oil inexpensively available 10-undecenoic relatively easily accessible.
Biochemically, docosanedioic acid is formed during the ω-oxidation of n -docosanoic acid ( behenic acid ) in the microsomes from rat liver cells via the intermediate 22-hydroxydocosanoic acid.
properties
When recrystallized from methanol, 1,22-docosadioic acid forms fine, colorless crystal needles with a waxy consistency. With its long C 20 hydrocarbon chain between the two functional end groups, 1,22-docosadioic acid is a distinctly hydrophobic spacer for chemical and biochemical conjugates.
Applications
One such application of the hydrophobic docosanedioic acid as a spacer between two 17 β -estradiol units leads to Estradioldimeren high strength of binding to specific estrogen receptors (ERa).
Phellogenic natural origin from beach Lilac (Limonium sp.) Acts as an inhibitor for the enzyme tyrosine phosphatase (PTP1B), which is an important negative regulator of the insulin receptor.
By reaction z. B. with N-hydroxysuccinimide under suitable conditions, a monosubstituted imide ester (active ester or NHS ester) of docosanedioic acid is obtained, which is used to modify enzymes and extend the plasma half-life of the biologically active proteins derivatized in this way and reduce their antigenicity.
Surface-active compounds of the ABA molecule type are obtained by reacting the activated carboxyl end groups of docosanedioic acid (building block A) with monomethoxy polyethylene glycols (building blocks B),
which are ascribed useful as solubilizers, wetting agents and embedding materials for drug delivery.
Docosanedioic acid can be used to stabilize microparticles with a biodegradable matrix of starch by crosslinking and hydrophobizing them (together with gas) as ultrasound contrast media.
Individual evidence
- ↑ a b c d S. Hünig, E. Lücke, W. Brenninger: Docosanedioic Acid In: Organic Syntheses . 43, 1963, p. 34, doi : 10.15227 / orgsyn.043.0034 ; Coll. Vol. 5, 1973, p. 533 ( PDF ).
- ↑ a b c d Data sheet Docosanedioic acid, 85% from Sigma-Aldrich , accessed on October 27, 2014 ( PDF ).
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . Elsevier, 2014, ISBN 978-0-323-28659-6 , pp. 343 .
- ↑ a b c Artur Seher: The constitution of isanoic and isanolic acid . In: Liebigs Ann. Chem. Band 589 , no. 3 , October 30, 1954, p. 222-238 , doi : 10.1002 / jlac.19545890308 ( PDF ).
- ↑ a b Entry on Japan wax. In: Römpp Online . Georg Thieme Verlag, accessed on October 27, 2014.
- ↑ a b Patent EP0511600 : Long chain carboxylic acid imide ester. Applied on April 24, 1992 , published on November 4, 1992 , Applicants: Kuraray Co., Ltd., Inventors: I. Ebashi, T. Takigawa, M. Inoue.
- ↑ a b Patent US5558857 : Contrast agents. Applied June 3, 1992 , published September 24, 1996 , Applicant: Nycomed Imaging AS, Inventors: J. Klaveness, P. Rongved, P. Strande.
- ↑ Fritz Zetzsche, Moritz Bähler: Investigations on the cork VII. Phellogenic acid (contribution to the behavior of the α-oxy acids in the potash melt) . In: Helv. Chim. Acta . tape 14 , no. 4 , July 1, 1931, p. 852–856 , doi : 10.1002 / hlca.19310140425 ( PDF ).
- ^ MC Garcia-Vallejo, E. Conde, E. Cadahia, B. Fernández de Simón: Suberin composition of reproduction cork from Quercus suber . In: wood research . tape 51 , no. 3 , 2009, p. 219–224 , doi : 10.1515 / ed . 1997.51.3.219 .
- ↑ Patent EP0974338 : Care cosmetic and dermatological preparations with a content of fatty acids. Registered on July 13, 1999 , published on January 26, 2000 , applicant: Beiersdorf AG, inventor: G. Lanzendörfer, V. Schreiner, G. Schneider, F. Wolf.
- ^ S. Hünig, E. Lücke, W. Brenninger: 1-Morpholino-1-cyclohexene In: Organic Syntheses . 41, 1961, p. 65, doi : 10.15227 / orgsyn.041.0065 ; Coll. Vol. 5, 1973, p. 808 ( PDF ).
- ^ ED Bergmann, R. Ikan: 6-Phenylazulene . In: J. Am. Chem. Soc. tape 78 , no. 7 , 1956, pp. 1482–1485 , doi : 10.1021 / ja01588a056 .
- ↑ RJ Sanders, R. Ofman, F. Valianpour, S. Kemp, RJ Wanders: Evidence for two enzymatic pathways for omega-oxidation of docosanoic acid in rat liver microsomes . In: J. Lipid Res. Volume 46 , no. 5 , 2005, p. 1001-1008 , doi : 10.1194 / jlr.M400510-JLR200 .
- ↑ AE Wendlandt, SM Yelton, D. Lou, DS Watt, DJ Noonan: Synthesis and functional analysis of novel bivalent estrogens . In: Steroids . tape 75 , no. 12 , 2010, p. 825-833 , doi : 10.1016 / j.steroids.2010.05.019 .
- ↑ Baumgartner: Virtual and Real Screening of Natural Products to Find Effective Modulators of Protein Tyrosine Phosphatase PTP1B. In: Scientia Pharmaceutica. 77, 2009, pp. 199-199, doi: 10.3797 / scipharm.oephg.21.SL-32 .
- ↑ J. Arukwe, B. Balinov, G. Hagelin, H. Dugstad, T. Thomassen: Synthesis and characterization of new monomethoxypoly (ethylene glycol) (mPEG) carbonate ester surfactants . In: Acta Chem. Scand. tape 53 , 1999, pp. 594-601 ( PDF ).




