10-undecinic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 10-undecinic acid | ||||||||||||||||||
| Molecular formula | C 11 H 18 O 2 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 182.26 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.9286 g cm −3 at 23.56 ° C |
||||||||||||||||||
| Melting point | |||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
10-Undecinic acid is a long-chain, linear unsaturated carboxylic acid with a terminal ethynyl group , which can be produced from 10-undecenoic acid, which is easily accessible from castor oil , by bromination and dehydrobromination and has fungicidal properties.
Manufacturing
10-Undecinic acid is obtained by adding bromine to the terminal double bond of 10-Undecenoic acid and then splitting off hydrogen bromide twice with sodium amide in liquid ammonia after fractional distillation and two recrystallization from petroleum ether in a yield of 38-42% of theory. receive.
According to a similar process variant, the pure yield after evaporation of the ammonia and one-time recrystallization from hexane is over 85% of theory.
The dehydrobromination also provides in a much simplified manner under phase transfer conditions with Aliquat 336 ( trioctylmethylammonium chloride ) and powdered sodium hydroxide in 1,2-dimethoxyethane 10-undecinic acid with a yield of 81% of theory. A similar process is also described in polyethylene glycol as a solvent with quantitative yield.
properties
10-Undecinic acid is a white to pale yellow colored, odorless and crystalline solid that dissolves only very little in water at room temperature. In contrast, the acid is readily soluble in short-chain alcohols such as methanol, ethanol, isopropanol, as well as in dichloromethane and DMSO . 10-undecinic acid is referred to as a natural fungicide (" a natural fungicide ") without any evidence (the fungicidal effect of 10-undecinic acid, however, has been extensively documented). In the European Union and in Switzerland, 10-undecinic acid is not approved as a crop protection agent.
Applications
10-Undecinic acid is suitable for stabilizing gold nanoparticles by neutralizing a gold (III) chloride solution to which it has been added and then heating it, whereby the typical bright red color of colloidal gold appears.
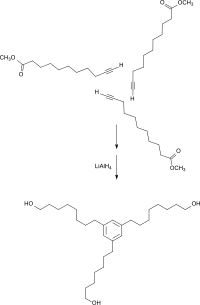
10-undecynoic acid can be esterified with methanol to obtain 10-undecynoic acid methyl ester , which is converted in a thiol-in coupling reaction with mercaptoethanol to give 10,11-bis (hydroxyethylthio) undecanoic acid methyl ester and after reduction of the methyl ester group as a polyol component for polyurethanes can be used with good biocompatibility.
The methyl ester can also be converted into a star-shaped triol of interest for segmented polyurethanes by transition metal-catalyzed cyclotrimerization and subsequent hydrogenation of the methyl ester function.
11-iodo-10-undecynoic acid, which is formed by iodination of 10-undecynoic acid in alkaline, as well as its zinc salt and phenyl ester show up to 100 times more fungicidal effect than 10-undecenoic acid.
Via its reactive ethynyl group, 10-undecinic acid can be linked in an asymmetric acetylene coupling Cadiot-Chodkiewicz coupling with bromoalkynols in the presence of copper (I) chloride to form long-chain ω-hydroxycarboxylic acids. the bromoalkynols required for this can be prepared by bromination of the ethynyl group with hypobromite .
With 1-bromopentyn-5-ol, 14-hydroxy-10,12-hexadecadiinic acid is obtained in a yield of 75% of theory obtained and can be hydrogenated on a platinum contact with 98% yield to the saturated ω-hydroxyhexadecanoic acid.
Long-chain substituted, disk-shaped mesogens as the base body of columnar liquid crystal phases can be obtained in a Steglich esterification with dicyclohexylcarbodiimide / 4- (dimethylamino) pyridine of 10-undecinic acid with a hydroxytriphenylene compound. A star-shaped trimer which has interesting liquid-crystalline properties can be formed from the ester obtained by means of a dicobalt octacarbonyl -catalyzed [2 + 2 + 2] cycloaddition .
10-Undecinic acid can be used as an anchor for functional molecules on carbohydrates, such as. B. Serve strength . The enzymatic esterification catalyzed by lipolase yields starch 10-undecinoate, to whose terminal ethynyl group via a Cu (I) -catalyzed azide / alkyne cycloaddition (see also click chemistry ), azide-functionalized fluorescent labels or azide-derivatized biotin are bound, and the labeled streptavidin is added able to bind.
10-undecinic acid is the starting material for pheromone syntheses, e.g. B. the insect attractant dodec-10E-enyl acetate.
Individual evidence
- ↑ a b c d N.A. Khan: 10-undecynoic acid In: Organic Syntheses . 32, 1952, p. 104, doi : 10.15227 / orgsyn.032.0104 ; Coll. Vol. 4, 1963, p. 969 ( PDF ).
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . Elsevier, 2008, ISBN 978-0-8155-1596-8 , pp. 315 .
- ↑ a b c d data sheet 10-Undecynoic acid from Sigma-Aldrich , accessed on October 20, 2014 ( PDF ).
- ↑ a b Data sheet 10-Undecynoic acid, 96% from AlfaAesar, accessed October 25, 2014 ( PDF )(JavaScript required) .
- ↑ a b Patent WO2007138345 : Process for producing stabilized metal nanoparticles. Filed May 22, 2007 , published December 6, 2007 , applicant: Johnson Matthey Plc., Inventor: PT Bishop, A. Boardman.
- ↑ a b B. Han, P. Hu, B.-Q. Wang, C. Redshaw, KQ Zhao: Triphenylene discotic liquid crystal trimers synthsized by Co 2 (CO) 8 -catalyzed terminal alkyne [2 + 2 + 2] cycloaddition . In: Beilstein J. Org. Chem. Volume 9 , 2013, p. 2852-2861 , doi : 10.3762 / bjoc.9.321 .
- ^ L. Brandsma: Preparative Acetylenic Chemistry . In: Studies in Organic Chemistry 34 . Elsevier, 1988, ISBN 0-444-42960-3 , pp. 173-174 .
- ^ A b D. Villemin, P. Cadiot, M. Kuetegan: A new synthesis of ω-hydroxyalkanoic acids via copper catalysis . In: Synthesis . 1984, ISSN 0039-7881 , p. 230-231 .
- ↑ S. Narasimhan, H. Mohan, N. Palani: An improved procedure for the synthesis of terminal and internal alkynes from 10-undecenoic acid . In: Synth. Commun. tape 21 , no. 18-19 , 1991, pp. 1941-1949 , doi : 10.1080 / 00397919108021786 .
- ↑ Santa Cruz Biotechnology, Inc .: 10-Undecynoic acid
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national directory of plant protection products in Switzerland ; Retrieved June 25, 2016.
- ^ RJ González-Paz, G. Lligadas, JC Ronda, M. Galià, V. Cádiz: Thiol-yne reaction of alkyne-derivatized fatty acids: biobased polyols and cytocompatibility of derived polyurethanes . In: Polym. Chem. Band 3 , 2012, p. 2471-2478 , doi : 10.1039 / C2PY20273E .
- ↑ G. Lligadas, JC Ronda, M. Galia, V. Cadiz: Polyurethane networks from fatty acid-based aromatic triols: synthesis, and characterization . In: Biomolecules . tape 8 , 2007, p. 1858–1864 , doi : 10.1021 / bm070157k .
- ↑ Patent US3420859 : 11-Iodo-10-undecynoic acid and is derivatives. Applied March 9, 1965 , published January 7, 1969 , Applicants: Kaken KK, Inventors: A. Ueno, E. Matsuzaki, Y. Momoki, Y. Ishimaru, G. Saito, S. Sakai.
- ↑ A. Alissandratos, N. Baudendistel, B. Hauer, K. Baldenius, S. Flitsch, P. Halling: Biocompatible functionalization of starch . In: Chem. Commun. tape 47 , 2010, p. 683-685 , doi : 10.1039 / C0CC02908D .
- ↑ RI Ishchenko, BG Kovalev, NB Kalyuzhnaya: Synthesis of dodec-10E-enylacetate - the sex pheromone of Phyllonorycter blancardella (Lepidoptera: Gracillariidae) . In: Chemistry of Natural Compounds . tape 32 , no. 1 , 1996, p. 77-79 , doi : 10.1007 / BF01373798 .