Diphenyl ether
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
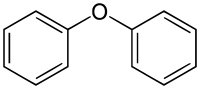
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diphenyl ether | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 10 O | |||||||||||||||
| Brief description |
colorless, flammable solid with a geranium-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 170.21 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.07 g cm −3 |
|||||||||||||||
| Melting point |
27 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
8 Pa (20 ° C) |
|||||||||||||||
| solubility |
almost insoluble in water (21 mg l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.579 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
1 ml m −3 or 7.1 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Diphenylether is a chemical compound from the group of ethers and the simplest diarylether . The compound consists of two benzene rings linked by an oxygen atom .
Extraction and presentation
Diphenyl ether and many of its properties were first published in early 1901. It is represented by a Ullmann condensation , through the reaction of phenol and bromobenzene in the presence of a base and a catalyst such as copper :
Due to side reactions, diphenyl ether is also a significant by-product in the high pressure hydrolysis of chlorobenzene in the production of phenol.
use
Diphenyl ether is used as a rose perfume for soaps and detergents and as a heat transfer medium. It is the starting material for many derived compounds and chemical syntheses. For example, its brominated derivatives ( polybrominated diphenyl ethers ) are used as flame retardants in many plastics and textiles. The aminofen derivative is used to produce diphenyl ether herbicides .
A eutectic mixture of biphenyl and diphenyl ether is offered as a heat transfer oil under the trade names Dowtherm A and Therminol VP-1 .
safety instructions
Diphenyl ether forms flammable vapor-air mixtures at high temperatures. With a flash point of 115 ° C, the substance is considered to be hardly inflammable. The explosion range is between 0.8% by volume (55 g / m 3 ) as the lower explosion limit (LEL) and 15% by volume (1060 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 610 ° C. The substance therefore falls into temperature class T1.
Individual evidence
- ↑ a b c d e f g h i j k l Entry on diphenyl ether in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 340, ISBN 3-342-00280-8 .
- ↑ Data sheet Diphenyl ether at AlfaAesar, accessed on June 25, 2016 ( PDF )(JavaScript required) .
- ↑ a b data sheet Diphenyl ether from Sigma-Aldrich , accessed on June 25, 2016 ( PDF ).
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 101-84-8 or diphenyl ether ), accessed on November 2, 2015.
- ^ Cook, AN: Derivatives of Phenylether . In: Journal of the American Chemical Society . 23, No. 11, 1901, pp. 806-813. doi : 10.1021 / ja02037a005 .
- ↑ Kürti; Czakó, Strategic Applications of Named Reactions in Organic Synthesis , 2005, p. 464.
- ↑ Fahlbusch, K.-G .; Hammerschmidt, F.-J .; Panten, J .; Pickenhagen, W .; Schatkowski, D .; Bauer, K .; Garbe, D .; Surburg, H .: Flavor and Fragrances . In: Wiley-VCH (Ed.): Ullmann's Encyclopedia of Industrial Chemistry . , Weinheim 2003. doi : 10.1002 / 14356007.a11_141 .
- ↑ DOWTHERM Synthetic Organic Fluids
- ↑ Therminol VP-1 Heat Transfer Fluid
- ^ E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.


