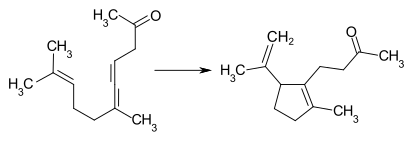
Pseudo-jonon
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Pseudo-jonon | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 13 H 20 O | ||||||||||||
| Brief description |
yellowish oil |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 192.30 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| density |
0.8951 g cm −3 (20 ° C) |
||||||||||||
| Melting point |
−75 ° C |
||||||||||||
| boiling point |
265.4 ° C |
||||||||||||
| Vapor pressure |
0.1741 Pa (20 ° C) |
||||||||||||
| solubility |
|
||||||||||||
| Refractive index |
1.5335 (20 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||
Pseudoionone is one of the terpenes scoring chemical compound . It is available as a yellowish oil.
Occurrence
Pseudojonon is a compound found in nature. It was found in tobacco plants . When digesting lycopene (e.g. from tomatoes), humans form pseudojonon. In plants, it is an intermediate in the biosynthesis of lycopene. Pseudojonon could therefore be found in many flowering plants and therefore widespread in nature.
Extraction and presentation
Since pseudojonon only occurs in small amounts in nature, it is almost exclusively produced synthetically.
For example, citral is condensed with acetone in an alkaline medium for representation .
Technically today, pseudojonone is usually produced from acetoacetic ester and dehydrolinalool by heating to 160 ° C. A mixture of two different diastereomers falls here .
A considerable part (up to 40%) of the intermediate product also cyclizes here . However, this can be prevented by adapting the reaction conditions.
Pseudojonon is one of the chemical substances that are produced in large quantities (" High Production Volume Chemical ", HPVC) and for which the Organization for Economic Cooperation and Development (OECD) collects data on possible hazards (" Screening Information Dataset ", SIDS ) was made.
properties
Chemical properties
In acidic solution, pseudo -jonone cyclizes to α- and β-ionone . When using phosphoric acid mainly α-ionone is formed; with sulfuric acid mainly β-ionone is formed.
use
Pseudo-jonon is mainly used for the synthesis of α- and β-ionone.
Biological importance
Due to its similar structure to juvenile hormone III, pseudojonone acts as a juvenile hormone in insects . These control the formation of the larval characteristics or determine the goal of the moult.
literature
- Lexicon entry: pseudojonon . In: Chemicals Lexicon. Status: January 6, 2000 (accessed on September 20, 2006)
- W. Walter, W. Francke, Textbook of Organic Chemistry , 23rd edition, 1998, S. Hirzel Verlag Stuttgart - Leipzig
Individual evidence
- ↑ a b c Entry on pseudo-jonon. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ a b c d e f g OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for 3,5,9-undecatrien-2-one, 6,10-dimethyl- , accessed on October 3, 2014.
- ↑ a b Data sheet Pseudoionone, technical, mixture of isomers, ≥90% (GC) from Sigma-Aldrich , accessed on May 29, 2011 ( PDF ).