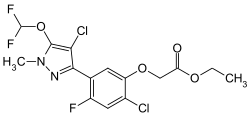
Pyraflufen-ethyl
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Pyraflufen-ethyl | ||||||||||||||||||
| other names |
Ethyl-2-chloro-5- (4-chloro-5-difluoromethoxy-1-methylpyrazol-3-yl) -4-fluorophenoxyacetate ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 15 H 13 Cl 2 F 3 N 2 O 4 | ||||||||||||||||||
| Brief description |
colorless odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 413.18 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.565 g cm −3 |
||||||||||||||||||
| Melting point |
126.4-127.2 ° C |
||||||||||||||||||
| Vapor pressure |
4.3 10 −9 Pa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Pyraflufen-ethyl is a chemical compound from the group of phenylpyrazoles .
Extraction and presentation
Pyraflufen-Ethyl can be obtained starting from 5- (ethoxycarbonylmethoxy) benzoylacetate by cyclocondensation with methylhydrazine to 3-aryl-5-hydroxypyrazole which is converted to the end product by O-difluoromethylation and chlorination with phosphorus pentachloride .
properties
Pyraflufen-Ethyl is a colorless, odorless solid that is practically insoluble in water. It is stable under acidic conditions, but hydrolyzes slowly under neutral and rapidly under alkaline conditions. It is derived as an ethyl ester from Pyraflufen (CAS number: 129630-17-7).
use
Pyraflufen-Ethyl is used as an active ingredient in crop protection products. It is used as a defoliant for cotton and potatoes and to control certain broad-leaved weeds in cotton, corn, soybeans, wheat and on non-agricultural land. It works by inhibiting protoporphyrinogen IX oxidase causing damage in cell membranes.
Admission
An application for approval of the compound in the European Union was submitted on June 16, 1997 in Belgium by the Nihon Nōyaku . Pyraflufen-Ethyl was approved for use as a herbicide in 2001. In Germany, Austria and Switzerland, plant protection products with this active ingredient are approved.
Web links
- Entry for Pyraflufen-ethyl in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed July 31, 2013.
Individual evidence
- ↑ a b c d e f g h i EU: Review report for the active substance pyraflufen-ethyl (PDF; 302 kB), July 2, 2002.
- ↑ a b Entry for CAS no. 129630-17-7 in the GESTIS substance database of the IFA , accessed on July 5, 2012(JavaScript required) .
- ↑ Entry on 2-chloro-5- (4-chloro-5-difluoromethoxy-1-methylpyrazol-3-yl) -4-fluorophenoxyacetic acid ethyl ester in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Pyraflufen-ethyl data sheet from Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ Peter Böger, Ko Wakabayashi: Peroxidizing Herbicides . Springer, 1999, ISBN 3-540-64550-0 , pp. 43 ( limited preview in Google Book search).
- ↑ CDPR: CALIFORNIA DEPARTMENT OF PESTICIDE REGULATION PUBLIC REPORT 2004-4 for Pyraflufen-ethyl (PDF; 94 kB).
- ↑ Directive 2001/87 / EC (PDF) of the Commission of October 12, 2001 amending Annex I of Directive 91/414 / EEC of the Council on the placing on the market of plant protection products containing the active substances acibenzolar-s-methyl, cyclanilide, iron ( III) phosphate, pymetrozine and pyraflufen-ethyl.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Pyraflufen-ethyl in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.
