Quizalofop-ethyl
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
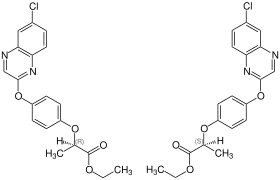
| Stereoisomers of quizalofop-ethyl: ( R ) -form (left) and ( S ) -form (right) | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Quizalofop-ethyl | ||||||||||||||||||
| other names |
( RS ) -2- [4- (6-chloroquinoxalin-2-yloxy) phenoxy] propionic acid ethyl ester ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 19 H 17 ClN 2 O 4 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 372.08 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.35 g cm −3 |
||||||||||||||||||
| Melting point |
76-78 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Quizalofop-ethyl is a chemical compound from the group of aryloxyphenoxypropionic acid - herbicides (a subgroup of the derivatives of phenoxyacetic acid and quinoxaline ).
Quizalofop-ethyl is a representative of the quizalofop herbicides , structurally it is the ethyl ester of quizalofop, the free acid.
Extraction and presentation
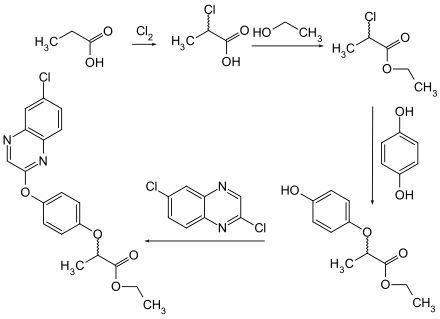
Quizalofop-ethyl can be obtained from propionic acid. This is chlorinated to 2-chloropropionic acid and then esterified with ethanol . The intermediate product then reacts with hydroquinone and 2,6-dichloroquinoxaline to form the end product.
properties
Quizalofop-ethyl is a colorless solid that is insoluble in water. It is unstable when exposed to light.
Stereoisomerism
Quizalofop-ethyl has one center of chirality and can therefore exist in two mirror-image forms, the ( R ) and the ( S ) isomer. The technical synthesis product is a racemic mixture of both isomers. The more effective ( R ) -isomer is called quizalofop-P-ethyl.
effect
The effect of quizalofop-ethyl is based on the inhibition of fatty acid - biosynthesis by engagement in the mode of action of the enzyme acetyl coenzyme A carboxylase .
Use and approval status
Quizalofop-ethyl is next quizalofop as a selective post-emergence - herbicide used that, but not against annual and perennial grasses against sedges used and broadleaf weeds. It was approved in the USA in the late 1980s.
Quizalofop-P-ethyl was approved for use as a herbicide in the EU with effect from December 1, 2009. Plant protection products with Quizalofop-P are only available in Switzerland, but not in Germany or Austria.
Individual evidence
- ↑ a b c d e f data sheet Quizalofop-p-ethyl, PESTANAL at Sigma-Aldrich , accessed on May 19, 2017 ( PDF ).
- ↑ a b c Entry on QUIZALOFOP-ETHYL in the Hazardous Substances Data Bank , accessed on July 29, 2012.
- ^ A b c Terence Robert Roberts, David Herd Hutson: Metabolic Pathways of Agrochemicals: Herbicides and plant growth regulators . Royal Society of Chemistry, 1998, ISBN 0-85404-494-9 , pp. 124 ( limited preview in Google Book search).
- ↑ Rudolf Heitefuss: Plant protection: Basics of the practical Phytomedicin . Georg Thieme Verlag, 2000, ISBN 3-13-513303-6 , p. 211 ( limited preview in Google Book search).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 776 ( limited preview in Google Book search).
- ↑ a b EPA: Quizalofop Summary Document ( Memento of April 12, 2013 in the Internet Archive ). December 2007.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Quizalofop-P-ethyl in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 23, 2018.