Rhenium (V) chloride
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
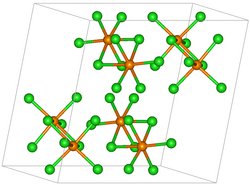
|
||||||||||||||||
| __ Re 5+ __ Cl - | ||||||||||||||||
| Crystal system |
monoclinic |
|||||||||||||||
| Space group |
P 2 1 / c |
|||||||||||||||
| Lattice parameters |
a = 924 pm, b = 1154 pm, c = 1203 pm, β = 109.1 ° |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Rhenium (V) chloride | |||||||||||||||
| other names |
Rhenium pentachloride |
|||||||||||||||
| Ratio formula | ReCl 5 | |||||||||||||||
| Brief description |
dark green solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 363.47 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.9 g cm −3 |
|||||||||||||||
| Melting point |
261 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−373 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Rhenium (V) chloride is an inorganic chemical compound of rhenium from the group of chlorides .
Extraction and presentation
Rhenium (V) chloride can be obtained by reacting rhenium with chlorine .
It can also be made from rhenium (VII) oxide and carbon tetrachloride .
properties
Rhenium (V) chloride is in the form of paramagnetic , black-brown, greenish shimmering crystals, which are unstable in humid air. The steam is also dark brown. It has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) and the lattice parameters a = 924 pm, b = 1154 pm, c = 1203 pm and β = 109.1 °. The crystal structure consists of Re 2 Cl 10 units with double-hexagonal packing. It is soluble in cyclohexane and reacts with oxygen at elevated temperatures to form rhenium oxide chlorides. It reacts with water to form HReO 4 and black insoluble rhenium (IV) oxide or green dissolved ReCl 6 2− . Can be distilled without decomposition in a chlorine atmosphere or in a vacuum, decomposes above the melting point in nitrogen or argon to form rhenium (III) chloride and chlorine.
use
Rhenium (V) chloride is used as a starting material for rhenium porphyrins and rhenium alkyne complexes.
Individual evidence
- ↑ a b c d e data sheet Rhenium (V) chloride from Sigma-Aldrich , accessed on May 25, 2017 ( PDF ).
- ↑ a b Roger Blachnik (Ed.): Pocket book for chemists and physicists . Volume III: Elements, Inorganic Compounds and Materials, Minerals . founded by Jean d'Ans, Ellen Lax. 4th, revised and revised edition. Springer, Berlin 1998, ISBN 3-540-60035-3 , pp. 694 ( limited preview in Google Book search).
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 2: Subgroup elements, lanthanoids, actinides, transactinides. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049590-4 , p. 1921 (Reading sample: Part C - Subgroup elements. Google book search ).
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1608.

