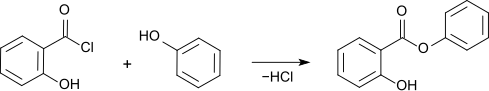
Salicylic acid phenyl ester
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Salicylic acid phenyl ester | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 13 H 10 O 3 | |||||||||||||||||||||
| Brief description |
white solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 214.22 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
41-43 ° C |
|||||||||||||||||||||
| boiling point |
172–173 ° C (16 h Pa ) |
|||||||||||||||||||||
| solubility |
bad in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Salicylic acid phenyl ester or phenyl salicylate is the phenyl ester of salicylic acid .
presentation
Phenyl salicylate can be prepared by reaction of Salicylsäurechlorid with phenol can be produced.
properties
Phenyl salicylate is a colorless, crystalline powder that has a slightly aromatic odor and is only very slightly soluble in water, but more soluble in benzene , chloroform , ethanol and ether .
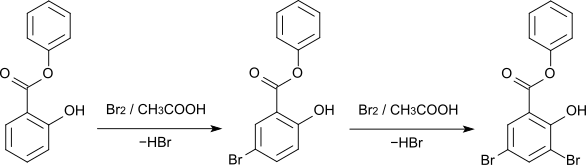
The bromination of phenyl salicylate with elemental bromine in glacial acetic acid initially leads to the 5-bromo derivative. The 3,5-dibromo derivative is formed when 2 moles of bromine are used.
use
Phenyl salicylate has an antiseptic and anti-rheumatic effect and is also used as UV -protection for the skin and for plastics use. It was introduced into therapy in 1886 as Salol ® ; The main purpose was to disinfect the urinary tract .
Individual evidence
- ↑ Entry on PHENYL SALICYLATE in the CosIng database of the EU Commission, accessed on May 22, 2020.
- ↑ a b c d e data sheet Phenyl salicylate from Sigma-Aldrich , accessed on April 22, 2011 and January 17, 2012 ( PDF ).
- ↑ Data sheet salicylic acid phenyl ester from Acros, accessed on April 2, 2010.
- ↑ Günter Jeromin: "Organic Chemistry: A Practice-Related Textbook", Harri Deutsch Verlag, 2006, p. 294, ISBN 978-3-8171-1732-1 . ( limited preview in Google Book search).
- ↑ P. Kauschke: "About the action of bromine on salicylic and benzoic phenyl, benzoic o-, m- and p-cresyl, and benzoic guaiacol" in J. Prakt. Chem. 1895 , 51 , p. 210ff.
- ↑ Entry on salicylic acid esters. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ^ Harry Auterhoff , Textbook of Pharmaceutical Chemistry, Wissenschaftliche Verlagsgesellschaft Stuttgart, 1968.