Salinity
As salinity (from latin Salinitas ) is called (simplifying) the salt content of a body of water , body of water , or water . In the simplest case, it is given as a mass fraction in g / kg (grams of salt per kilogram of salt water or solution) or in percent (1% corresponds to 10 g / kg).
The salinity can be determined with the help of a salinometer . This makes use of the fact that the electrical conductivity of the water is proportional to the salt content. In oceanography , salinity is an important parameter when determining water masses and ocean currents .
History of salinity determination
With the salinity determination one wants to find out how much salt is dissolved in a quantity of water. The salt content, together with the pressure and the temperature, is responsible for the density or the potential density of the water. This means that different salt contents have a major impact on ocean currents.
evaporation
The most primitive method is to evaporate water and weigh what has not evaporated. The problem is that water can become trapped in salt crystals. In order to dissolve this water from the salt crystals, high temperatures are necessary. Due to the high temperature, however, some salts decompose (e.g. MgCO 3 → MgO + CO 2 ). Robert Boyle already established in the 17th century that the drying and weighing of seawater led to poorly reproducible results for the concentration of dissolved substances.
In the 19th century, the Marcet principle or the law of constant proportions was formulated. It says: Regardless of how much the salinity differs from place to place, the proportions of the most important ions (English major ions) in the water of the open ocean are almost constant.
At the beginning of the 20th century, new methods of determining the salt content appeared.
Salinity, S ‰ ( Knudsen , 1902)
In order to get reproducible results, a first strict definition was introduced. "Salinity is defined as the weight in grams of the dissolved inorganic substances in one kilogram of seawater after all bromides and iodides have been replaced with the same amount of chlorides and all carbon compounds have been oxidized." all carbon compounds are emitted as CO 2 . For this purpose, the sea water, HCl and saturated chlorine water were dried, heated for 72 hours at 480 ° C. and finally the residual chloride was titrated.
Although this method brought reproducible results, it was very cumbersome and only suitable to a limited extent for use at sea.
Chlorinity, Cl ‰ (Sørensen and Knudsen, 1902)
Using the law of constant proportions, it is possible to estimate the content of the remaining salts very precisely from the content of one salt. With the help of Mohr titration , the amount of halides in seawater could be determined. When used with seawater, silver bromide and silver iodide precipitate in addition to silver chloride, which can now be weighed. So that the results are precise , the silver nitrate solution is calibrated against so-called standard sea water with a known chlorinity. In order to calculate the salinity from the determined chlorinity, Sørensen measured the salinity of nine seawater samples directly and also determined the chlorinity. The correlation derived from this was: Salinity [‰] = 1.805 · Chlorinity [‰] + 0.030
This method is very precise and also much more applicable at sea than that of 1902.
Chlorinity, Cl ‰ ( UNESCO , 1962)
However, as the addition of a constant value in the above correlation suggests, there was a problem. Some of the nine samples came from the Baltic Sea. However, the Baltic Sea has a different ionic composition than the open ocean. This error was corrected by a new calibration now after 60 years: Salinity [‰] = 1.80655 · Chlorinity [‰]
Practical Salinity Scale, S (1978)
The Practical Salinity Scale (PSS-78) used today is based on the proportionality of salinity and electrolytic conductivity and is dimensionless. However, a PSU, which stands for Practical Salinity Unit, is often found below to indicate the salinity. This is not a physical unit.
The following formula is used to calculate the salt content from the conductivity:
K 15 indicates the ratio of the measured conductivity to the conductivity of a potassium chloride solution of 32.4356 g / kg at 15 ° C and at a pressure of one bar. If the ratio K 15 is equal to one, then S = 35.
Nowadays, conductivity is usually measured automatically with the help of a Conductivity Temperature Depth probe ( CTD ), in German a conductivity temperature depth probe .
Fluctuations in salinity in water
Short-term fluctuations
Due to the influence of weather and tides , the salinity is subject to natural fluctuations. On the one hand, the increase in salinity due to evaporation at low tide in areas near the shore ( Wadden Sea , tidal pools ) up to the formation of salt meadows , for example through longer periods of sunshine after storm surges . If the soil layer is impermeable to water, flat groundwater with a high level of salinity can form in marshland , which on the island of Læsø in the Kattegat reaches a salinity of up to 15 percent. A reduction in salinity can result from fresh water entering river mouths, meltwater zones and heavy rain .
Even when sea water freezes, salt remains. Although the salt is initially in the ice after freezing, a large part of it diffuses out and returns to the sea water, the salt content of which increases as a result. [Receipt?]
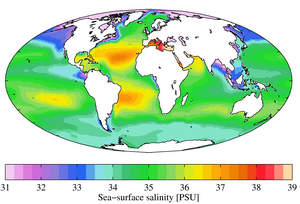
Large-scale differences
The salinity at the water surface typically differs from sea to sea. In the Baltic Sea region, where precipitation (including the land area from which the Baltic Sea is fed) is significantly greater than evaporation, the surface water is diluted. The further east you go, the lower the surface salinity. The dilution of the surface water leads to a stable water stratification, where low-salt water from the Baltic Sea lies over higher-salt water from the North Sea.
The opposite happens in the Mediterranean Sea - there evaporation is greater than the amount of precipitation. The salt remains in the water, its content increases. If the Mediterranean water emerges from the Mediterranean near Gibraltar, it sinks to medium depths despite its high temperature. Due to its high salt content, it can be identified almost throughout the North Atlantic.
Salinity
| The ten main components of sea salt (in%) |
|
|---|---|
| chloride | 55.04 |
| sodium | 30.61 |
| sulfate | 7.68 |
| magnesium | 3.69 |
| Calcium | 1.16 |
| potassium | 1.10 |
| Bicarbonate | 0.41 |
| bromide | 0.19 |
| Borate | 0.07 |
| strontium | 0.04 |
The most important dissolved salt ions in seawater have the same share of salinity in the world's oceans; they are conservative. This means that the proportion of ions to one another is the same even if the salinity is different. This is because once they have entered the sea they are no longer significantly influenced by biological or geochemical processes. A simple conceptual classification is: "Water can be polyhaline (over 10 per thousand salts - marine animals only), mesohaline (1.0 to 10 per thousand salts - special brackish water fauna) or oligohaline (0.1 to 1.0 per thousand salts - even freshwater animals) "
classification
- Fresh water has a salinity of less than 0.1% (i.e. less than 1 g / kg.)
- When brackish water salinity is between 0.1% and 1.0%.
- From a salinity of over 1.0% one speaks of salt water .
For comparison:
- The average salinity of the oceans is around 3.5% (35 g / kg).
- An isotonic saline solution contains 0.9% (9 g / kg) saline .
- A saturated saline solution contains 35.6% (356 g / kg) sodium chloride at 0 ° C (359 g / kg at 25 ° C).
Oceans
- Atlantic : 3.54%
- Indian Ocean : 3.48%
- Pacific : 3.45%
More seas
- Baltic Sea : 0.8%
- Bay of Kiel : 1.5%
- Finnish and Botnia : 0.1%
-
North Sea : 3.5%
- Estuaries: 1.5% - 2.5%
- Northern North Sea: 3.2% - 3.5%
- Mediterranean : 3.74%
- Black Sea 1.7% - 1.8%
- Persian Gulf : 4%
- Red Sea : 4%
The salinity of the other secondary seas is between 3% and 4%.
Inland lakes
- Caspian Sea : 1.3%
- Mono Lake : 7.3%
- Qarun Lake : 11.8%
- Dead Sea : 28% (average)
- Dead Sea: 32.66% at a depth of 50 meters
- Aral Sea : 0.9% (1960)
- Small Aral Sea: 2% (2003)
- Great Aral Sea: 7.5% (2003)
- Assalsee : 34.8% (average)
- Assalsee: 38.8% at a depth of 20 meters
- Don Juan Lake : 44.2% (world's highest value for a body of water)
See also
- Abiotic environmental factors , ecology
- Isotonic saline solution (physiological saline solution)
- SMOS
- Water hardness
Web links
- International oceanographic tables. (PDF; 7.3 MB) UNESCO (English)
- Salinometry.com - website with literature references, collection of methods, calculation aids and further information
- Equations and algorithms for fundamental properties of sea water according to UNESCO standards.
- Salt map of the seas
Individual evidence
- ^ Günter Dietrich, Kurt Kalle, Wolfgang Krauss, Gerold Siedler: Allgemeine Meereskunde. 3. Edition. Borntraeger, Berlin 1975. ISBN 3-443-01016-4 .
- ↑ Temperature And Salinity Scales ( Memento from January 11, 2006 in the Internet Archive )
- ^ T. Dauphinee: Introduction to the Special Issue on the Practical Salinity Scale 1978 . In: IEEE Journal of Oceanic Engineering . tape 5 , no. 1 , 1980, p. 1–2 ( PDF - Introduction to the special edition on the PSS-78 - free full text).
- ^ Joseph Moran: Ocean studies: Introduction to oceanograpy . Ed .: American Meteorological Society. 3. Edition. American Meteorological Society, Boston 2011.